Cancer Treatments - vitamin D is one of the 30 alternatives
THE ROLE OF REPURPOSED DRUGS AND METABOLIC INTERVENTIONS IN TREATING CANCER
Paul E. Marik, MD, FCCM, FCCP. © 2020-2023 FLCCC Alliance.
📄 Download the 125 page PDF from VitaminDWiki
PDF Table of contents
CHAPTER 1: INTRODUCTION 7
THE SOCIETAL IMPACT OF CANCER 7
CHAPTER 2: WHAT IS CANCER: UNDERSTANDING ITS PATHOGENETIC CAUSES 9
AN ALTERNATE THEORY: CANCER IS A METABOLIC DISEASE 10
CANCER SIGNAL PATHWAYS 13
CANCER IMMUNITY 15
PLATELETS AND CANCER 20
ANGIOGENESIS AND METASTASIS 20
CANCER STEM CELLS (CSC) 20
CHAPTER 3: PREVENTING CANCER 23
CHAPTER 4: THE METABOLIC APPROACH TO TREATING CANCER 25
DIETARY CALORIC RESTRICTION, THE KETOGENIC DIET, AND "REAL" FOOD 26
MANAGEMENT OF CANCER CACHEXIA 29
INTERMITTENT FASTING, AUTOPHAGY, AND CANCER 30
INSULIN POTENTIATION THERAPY FOR CANCER? 32
CHAPTER 5: REPURPOSED DRUGS FOR METABOLIC CANCER TREATMENT 34
SUMMARY OF TOP METABOLIC INTERVENTIONS TO CONTROL CANCER 35
METRONOMIC DOSING 36
DETAILED DESCRIPTIONS 37
Glucose management 37
Green Tea 42
Melatonin 44
Vitamin D 47
Metformin 54
Curcumin 55
Mebendazole/ Fenbendazole/Albendazole 59
Berberine 61
Atorvastatin 62
Stress Reduction and Exercise (aerobic and resistance training) 63
Phosphodiesterase 5 inhibitors: sildenafil, tadalafil, and vardenafil 64
Cimetidine 65
Doxycycline 67
Resveratrol 69
Cyclooxygenase inhibitors - Aspirin (ASA) and NSAIDs (Diclofenac) 70
Nigella sativa 75
Ganoderma lucidum (Reishi) and other medicinal mushrooms 76
Ivermectin 77
Dipyridamole 79
High dose intravenous vitamin C 80
Dichloroacetate (DCA) 81
CHAPTER 6: POTENTIAL ADJUNCTIVE THERAPIES 83
TUMOR TREATING FIELDS 83
PHOTODYNAMIC THERAPY 83
HYPERBARIC OXYGEN THERAPY 84
Cancer snips from PDF
In 2000, only two oncology drugs garnered more than $1 billion in sales. Just ten years later, the top 10 oncology drugs each exceeded $1 billion in revenue. By 2010, there were three oncology drug sales representatives for every 10 oncologists in the United States . Cancer, you see, is big business. (4) Patients and their families frequently face extreme financial burden and distress as a result of cancer treatment, this is known as “financial toxicity”. (5)
More recent data from the U.S. indicate that the 5-year cancer survival rate has only increased from 63% to 68% over the last 25 years (1995 to 2018).
The U.S. Department of Defense Medical Epidemiology Database (DMED) (41) reported a 664% increase in malignant neoplasms following the deployment of COVID-19 mRNA vaccination in the military (until this data was erroneously removed).
Vitamin D
Vitamin D is synthesized in human skin after the photoisomerization of 7- dehydrocholesterol to pre-vitamin D3 under the influence of UV B radiation (wavelength, 280-315 nm). The major factors influencing this process are either environmental (latitude, season, time of day, ozone and clouds, reflectivity of the surface) or personal (skin type, age, clothing, use of sunscreen, genetics). (286) From the skin, parental vitamin D3 (cholecalciferol) finds its way into the general circulation, and it is then metabolized in the liver to 25-hydroxyvitamin D3 [25(OH)D3] (calcifediol). 25(OH)D3 is an immediate precursor metabolite to the active form of vitamin D3, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (calcitriol), that is the product of the mitochondrial CYP27B1-hydroxylase confined primarily but not entirely to the proximal tubular epithelial cell of the kidney. (286, 287)
As vitamin D has a much shorter half-life than 25(OH)D3 (1-2 days versus 2-3 weeks), 25(OH)D3 is considered the best indicator of vitamin D status; hence 25(OH)D3 is the most widely used test indicating vitamin D status. (286, 287) A vitamin D level > 30 ng/ml is widely considered "normal" while a level between 20-30 ng/l is considered vitamin D insufficient and a level <20 ng/ml is considered vitamin D deficient. (286-288) However, more recent data suggests that a level > 50 ng/ml is desirable, and ideally targeting a level between 55- 90 ng/ml is desirable. (289-291)
It may take many months or even years to achieve optimal levels in patients with low vitamin D levels (< 20 ng/ml) taking the standard recommended dose of 5,000 lU/day. It is therefore important that the optimal regimen for vitamin D supplementation be followed to achieve adequate circulating levels (see Table 3). (290, 291) Since the highest dose of commercially available vitamin D3 is 50,000 IU capsules, and due to its affordability (low cost) and better gastrointestinal absorption, we recommend using 50,000 IU D3 capsules for community setups. Together, a number of these capsules can be taken as a bolus dose [i.e., single upfront doses such as 100,000 to 400,000 IU]. However, the liver has a limited 25- hydroxylase capacity to convert vitamin D to 25(OH)D: thus, taking 50,000 IU capsules over a few days provides better bioavailability.
Vitamin D2 is manufactured through the ultraviolet irradiation of ergosterol from yeast, while vitamin D3 is through the ultraviolet irradiation of 7-dehydrocholesterol from lanolin; both are used in over-the-counter vitamin D supplements. (286) Vitamin D2 has 30% of the biological activity of vitamin D3. It is best to include both Vitamin K2 (Menaquinone [MK7] 100 mcg/day, or 800 mcg/week) and magnesium (250-500 mg/day) when doses of vitamin D > 8 000 lU/day are taken. (292, 293) It should be noted that vitamin K2 itself has anticancer properties and an inverse relationship exists between vitamin K2 (and not K1) intake and cancer mortality. (294-297)
Table 3. Guidance on Upfront Loading Dose Regimens to Replenish Vitamin D Stores in the Body
When serum vitamin D levels are available, the doses provided in this table can be used for the longer-term maintenance of serum 25(OH)D concentration above 50 ng/mL (125 nmol/L). The table provides the initial bolus dose, weekly dose, frequency, and duration of administration of oral vitamin D in non-emergency situations, in a non-obese, 70 kg adult.
Serum Vitamin D (ng/mL) ** Vitamin D Dose: Using 50,000 IU Capsules: Initial and Weekly ® Duration (Number of Weeks) Total Amount Needed to Correct Vit. D, Deficiency (IU, in Millions) #
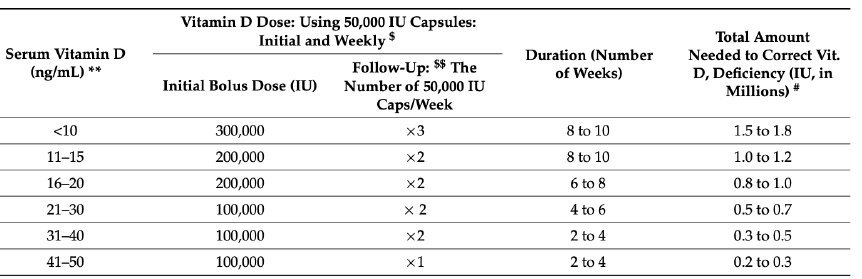
Table 3. Replenishing Vitamin D Stores (Source Nutrients - Special Issue: "Vitamin D - Calciferol and COVID" (290) Reproduced with permission from the author.
More than half of human tissues express the gene for the vitamin D receptor, with vitamin D having pleiotropic functions in pathways of energy metabolism, immunity, and cellular growth and differentiation that clearly extend the control of calcium homeostasis. (298) The most important extra-skeletal function of vitamin D is its role in the modulation of the immune system. Vitamin D receptors are present on immune cells, with this vitamin playing a critical role in both innate and adaptive host immunity. (299, 300)
Vitamin D has anticancer effects both directly via controlling the differentiation, proliferation, and apoptosis of neoplastic cells as well as indirectly through regulating immune cells that affect the microenvironment of malignant tumors. Evidence from observational and randomized controlled studies indicates that low vitamin D status is associated with higher mortality from life-threatening conditions such as cancer and cardiovascular disease. (301, 302) In a real-world analysis of 445,601 participants, aged 4073 years, from the UK Biobank cohort, both vitamin D deficiency and insufficiency were strongly associated with all-cause mortality. (303) A Cochrane analysis demonstrated that supplementation with vitamin D3 (cholecalciferol) decreased all-cause mortality (RR 0.94, 95% CI 0.91 to 0.98, p = 0.002); however, supplementation with vitamin D2, calcifediol, and calcitriol did not affect mortality. (304)
Vitamin D deficiency has been demonstrated to increase the risk of breast cancer while supplemental vitamin D intake had an inverse relationship with this outcome. (305) Both prospective and retrospective epidemiologic studies indicate that levels of 25- hydroxyvitamin D below 20 ng per milliliter are associated with a 30 to 50% increased risk of incident colon, prostate, and breast cancer, along with higher mortality from these cancers. (286) People living at higher latitudes are at increased risk for vitamin D deficiency and are reported to have an increased risk of Hodgkin's lymphoma as well as colon, pancreatic, prostate, ovarian, breast, and other cancers and are more likely to die from these cancers, as compared with people living at lower latitudes. (200, 286) Vitamin D supplementation likely plays an important role in the prevention of cancer, as highlighted in the prospective study by Bischoff-Ferrari et al (see section on Primary Cancer Prevention). (134, 135) Furthermore, in a meta-analysis of 50 trials with a total of 74,655 participants, Zhang et al reported that Vitamin D supplementation significantly reduced the risk of cancer death (0.85,0.74 to 0.97, 0%). (306) In subgroup analyses, all-cause mortality was significantly lower in trials with vitamin D3 supplementation than in trials with vitamin D2 supplementation. An analysis of 25(OH)D-cancer incidence rates suggests that achieving a vitamin D level of 80 ng/mL vs. 10 ng/mL would reduce cancer incidence rates by 70 ± 10%. (200)
Anticancer pathways and mechanisms
Experimental evidence indicates that vitamin D has diverse antineoplastic activity (see Figure 9). Binding of vitamin D to its target, the vitamin D receptor, leads to transcriptional activation and repression of target genes and results in induction of differentiation and apoptosis, inhibition of cancer stem cells, and decreased proliferation, angiogenesis, and metastatic potential. (307) Vitamin D induces apoptosis of cancer cells, (308) counteracts aberrant WNT-0 catenin signaling, (309) and has broad anti-inflammatory effects via downregulation of nuclear factor-K0 and inhibition of cyclooxygenase expression. (310) In colon, prostate, and breast carcinoma cells, 1,25-(OH)2D3 upregulates several pro-apoptotic proteins (BAX, BAK, BAG, BAD, G0S2) and suppresses survival and anti-apoptotic proteins (thymidylate synthase, survivin, BCL-2, BCL-XL). (311) In this way, it favors the release of cytochrome C from mitochondria and the activation of caspases 3 and 9 that lead to apoptosis. 1,25-(OH)2D3 and metformin have additive/synergistic antiproliferative and proapoptotic effects in colon carcinoma and other types of cells. (312)
In many cancer cell types, 1,25-(OH)2 D3 directly arrests the cell cycle In the G0/G1 phase by downregulating cyclin-dependent kinases and repressing genes that encode cyclins D1 and C. (313) 1,25-(OH)2D3 decreases the expression of epidermal growth factor receptor (EGFR) and interferes with the insulin-like growth factor (IGF)-I/II pathway. (200) Vitamin D has activity against human breast cancer cell lines by targeting Ras/MEK/ERK pathway. (311) In addition, 1,25-(OH)2D3 diminishes the proliferation of breast cancer cells by inhibiting estrogen synthesis and signaling through estrogen receptor (ER)a. (314) In colon carcinoma cells, 1,25-(OH)2 D3 upregulates an array of intercellular adhesion molecules that are constituents of adherens junctions and tight junctions, including E-cadherin, occludin, claudin-2 and -12, and ZO-1 and -2. (315) The Wnt/0-catenin pathway plays an important role in cancer. Antagonism of the Wnt/3-catenin pathway by 1,25-(OH)2 D3 was reported in colon carcinoma cells by a double mechanism: (a) liganded VDR binds nuclear 3-catenin, which hampers the formation of transcriptionally active |3-catenin/TCF complexes, and (b) induction E-cadherin expression that attracts newly synthesized 3-catenin protein to the plasma membrane adherens junctions. In that way, it decreases 3-catenin nuclear accumulation. (316)
1,25-(OH)2 D3 is an important modulator of the immune system, as reflected by the expression of vitamin D receptors by almost all types of immune cells. 1,25-(OH)2D 3 is an enhancer of innate immune reactions against tumor cells by activating macrophages, natural killer (NK) cells, and neutrophils. (200) An important mechanism of 1,25-(OH)2D3 is the inhibition of the NF-KB pathway. In turn, this causes the downregulation of multiple cytokines and their effects. 1,25(OH)2 D3 reduces the protumorigenic effect of PG E2 in prostate cancer cells by inhibiting COX-2 and so decreasing the levels of PG E2 and two PG receptors (EP2 and FP). (317)
Autophagy is a process of elimination of cytoplasmic waste materials and dysfunctional organelles that serves as a cytoprotective mechanism but that, when excessive, leads to cell death. (200) In cancer, VDR ligands trigger autophagic death by inducing crucial genes in several cancer cell types. Thus, 1,25-(OH)2 D3 de-represses the key autophagic MAP1LC3B (LC3B) gene and activates 50-AMP-activated protein kinase (AMPK). In Kaposi's sarcoma cells and myeloid leukemia cells, vitamin D compounds inhibit PI3K/AKT/mTOR signaling and activate Beclin-1-dependent autophagy. 1,25-(OH)2D3 has a pro-differentiation effect on several types of carcinoma cells either by direct upregulation of epithelial genes and/or the repression of key epithelial mesenchymal transcription factors (EMT-TFs). (318)
In diverse types of carcinoma cells (colon, prostate, and breast), the antiangiogenic action of 1,25-(OH)2 D3 relies to a great extent on its ability to inhibit two major angiogenesis promoters: it suppresses the expression and activity of hypoxia-inducible factor (HIF)-la, a key transcription factor in hypoxia-induced angiogenesis, and of vascular endothelial growth factor (VEGF). (200) 1,25-(OH)2D3 also has inhibitory effects on tumor-derived endothelial cells. It reduces their proliferation and sprouting in vitro and diminishes the blood vessel density in cancer models. (319)
Figure 9. Overview of metabolic pathways of Vitamin D. (Source: Dr. Mobeen Syed}
Footnote for Figure 9: CYP27A1: Cytochrome P450 family 27 subfamily A member 1, CYP27B1: Cytochrome P450 family 27 subfamily B member 1, 25(OH)D: 25-hydroxyvitamin D, 1,25(OH)2 D3: 1,25-dihydroxyvitamin D3, GC: Vitamin D-binding protein (Gc protein), VDR: Vitamin D receptor, RXR: Retinoid X receptor, VDRE: Vitamin D response element, CDKN1A: Cyclin-dependent kinase inhibitor 1A, C-MYC: Cellular Myelocytomatosis oncogene, CDH1: Cadherin-1, DKK1: Dickkopf-1, DKK4: Dickkopf-4, FOXM1: Forkhead box protein M1, LRP6: Low-density lipoprotein receptor-related protein 6, PI3K: Phosphatidylinositol 3-kinase, Akt: Protein kinase B, MEK: Mitogen-activated protein kinase kinase, ERK: Extracellular signal-regulated kinase, Rho A: Ras homolog gene family member A, ROCK: Rho-associated protein kinase, P38: p38 mitogen- activated protein kinase, MAPK: Mitogen-activated protein kinase, MSK1: Mitogen- and stress-activated protein kinase 1
Clinical studies
Data suggest that the majority of patients with cancer are vitamin D deficient (level < 20 ng/ml). (302, 307, 320, 321) Several prospective observational studies have shown that higher levels of plasma 25-hydroxyvitamin D were associated with improved survival among patients with colorectal cancer. (320, 322-324) Similarly, elevated 25-OH D levels were associated with better overall survival in patients with breast and gastric cancer and lymphoma. (325) In a population-based study of patients with cancer of the breast, colon, lung, and lymphoma a 25-OHD level below 18 ng/ml at diagnosis experienced shorter survival. (326) In a meta-analysis of 19 studies Robsahm et al reported an inverse relationship between 25-Hydroxyvitamin D and cancer survival. (327)
Chen performed a meta-analysis of observational cohort studies and randomized trials which assessed the role of post-diagnosis vitamin D supplement intake on survival among cancer patients. (328) The meta-analysis included 11 publications consisting of 5 RCTs and 6 observational cohort studies. The summary relative risk (SRR) for overall survival of vitamin D supplement use vs. non-use, pooling cohort studies and randomized trials, was 0.87 (95% CI, 0.78-0.98; p = 0.02). Vaughan-Shaw et al performed a meta-analysis of 7 studies evaluating the use of supplemental vitamin D in patients with colorectal cancer. (329) The study reported a 30% reduction in adverse outcomes and a beneficial effect on progressionfree survival (HR = 0.65; 95% CI: 0.36-0.94). In a meta-analysis by Kuznia et al, subgroup analysis revealed that vitamin D3 administered daily, in contrast to bolus supplementation, reduced cancer mortality by 12 %. (330) It should be recognized that a daily dose of between 800 IU and 4000 IU was administered in the studies included in this meta-analysis and that vitamin D levels were not monitored. A more dramatic reduction in mortality would likely be realized if patients were dosed more appropriately.
SUNSHINE was a double-blind, multicenter, randomized clinical trial designed to evaluate the efficacy of "high dose" vitamin D3 compared with standard-dose vitamin D3 given in combination with standard chemotherapy in patients with metastatic colorectal cancer. (307) The high-dose group received a loading dose of 8,000 IU per day of vitamin D3 (two 4,000 IU capsules) for cycle 1 followed by 4,000 IU/d for subsequent cycles. The standard dose group received 400 IU/d of vitamin D3 during all cycles. In this underpowered (n=139) RCT, multivariable HR for progression-free survival or death was 0.64 (95% CI, 0-0.90; p = .02) in favor of the high dose group. Comparison of progression-free survival between the high-dose and standard-dose vitamin D3 groups using a log-rank test stratified by ECOG performance status was statistically significant (p = .03). At baseline, median plasma 25- hydroxyvitamin D levels were deficient in both the high-dose vitamin D3 group (16.1 ng/mL [IQR, 10.1 to 23.0 ng/mL]) and in the standard-dose vitamin D3 group (18.7 ng/mL [IQR,
13.5 to 22.7 ng/mL]). Only 9% of the total study population had sufficient levels (>30 ng/mL) of 25-hydroxyvitamin D at baseline. At treatment discontinuation, patients in the high-dose vitamin D3 group had a median 25-hydroxyvitamin D level of 34.8 ng/mL (IQR, 24.9-44.7 ng/mL), whereas those in the standard-dose vitamin D3 group were still deficient in vitamin D and had a median 25-hydroxyvitamin D level of 18.7 ng/mL(IQR, 13.9-23.0ng/mL) (difference, 16.2 ng/mL [95% CI, 9.9-22.4 ng/mL]; P < .001). It is important to note that based on these levels the "high dose" group was profoundly underdosed. As indicated above, vitamin D dosing should be based on a serum level aiming for a level of > 50 ng/ml (target 55-90 ng/ml). Based on the data from this study we would suggest a daily dose of vitamin D3 of 20,000 to 50,000 lU/day until a vitamin D level is obtained. It is possible that patients with cancer may require an even higher level, approximating 150 ug/dl.
Wang et al demonstrated that postoperative vitamin D supplementation in esophageal cancer patients undergoing esophagectomy was associated with improved quality of life and with improved disease-free survival. (331) Similarly, vitamin D use post-diagnosis was found to be associated with a reduction in breast cancer-specific mortality. (332) Two recent clinical trials in prostate cancer patients suggest that vitamin D supplementation may prevent prostate cancer progression. (333, 334) Vitamin D has additive or synergistic effects when combined with conventional chemotherapy. (312) Zeichner et al demonstrated that use of vitamin D during neoadjuvant chemotherapy in HER2-positive nonmetastatic breast cancer was associated with improved disease-free survival (HR, 0.36; 95% Cl, 0.15-0.88; p=0.026). (335)
Types of cancers that Vitamin D may be beneficial for
Vitamin D supplementation is likely beneficial in most cancers, but particularly in patients with breast, colorectal, gastric, esophagus, lung, and prostate cancer as well as those with lymphomas and melanoma.
Dosing and cautions
As almost all patients with cancer are severely vitamin D deficient. A high loading dose of Vitamin D is suggested followed by dose titration according to vitamin D blood levels, aiming for a level of > 50 ng/ml (target 55-90 ng/ml). However current data suggest that levels up to 150 ng/mL are necessary for certain types of cancer to stop growth and metastasis. Vitamin D intoxication is observed when serum levels of 25-hydroxyvitamin D are greater than 150 ng per milliliter (374 nmol per liter). (286) Hypercalcemia will usually not occur until levels exceed over 250 ng/ml. We, therefore, suggest a daily dose of 20,000 to 50,000 lU/day until a vitamin D level is obtained. With the suggested doses, serum 25(OH)D concentrations rise above 100 ng/mL within a week or two, but unless a suitable higher maintenance dose is used (~ 10,000 lU/day), the level will start to drop to baseline after three weeks or so, and the benefit of vitamin D will be lost. If measuring vitamin D levels is not feasible/possible, we would suggest a loading dose of 100,000 IU followed by 10,000 lU/day. Doses of 10,000 IU of vitamin D3 per day for up to 5 months were reported to be safe and without toxicity. (286, 289) It should be noted that dosages of vitamin D up to 80,000 lU/day have been reported to be safe. (336, 337) We recommended vitamin D3 over D2 as vitamin D2 is approximately 30% as effective as vitamin D3 in maintaining serum 25-hydroxyvitamin D levels. (286) Furthermore, vitamin D3 should be dosed daily rather than large intermittent bolus dosing. It is best to include both Vitamin K2 (Menaquinone MK4/MK7 100 mcg/day, or 800 mcg/week) and magnesium (250-500 mg/day) when doses of vitamin D > 8 000 lU/day are taken. (292, 293) Patients taking coumadin need to be closely monitored and the need to consult with their PCP before taking vitamin K2. Further, we suggest measuring PTH (parathyroid) levels and calcium levels and titrating the dose of Vitamin D according to the PTH levels as follows (Coimbra Protocol): (338, 339) i) if the PTH level is below the lower end of the reference range, reduce the dose of Vitamin D ii) if the PTH level is at (or close too) the lower end of the reference range, maintain dose, iii) if PTH is within the reference range but not near to the low end of the reference range increase the dose of Vitamin D.
715 References from PDF
Cancer Facts & Figures 2023. Atlanta; 2023.
Hope JR. Surviving Cancer, COVID-19 & Disease. The repurposed drug revolution. Redding, CA: Hope Pressworks International; 2020.
Wulaningsih W, Garmo H, Holmberg L, Hammar N, Jungner I, Walldius G, et al. Serum Lipids and the Risk of Gastrointestinal Malignancies in the Swedish AMORIS Study. J Cancer Epidemiol. 2012;2012:792034.
Ten Trends Transforming The Business of Oncology. https://www.obroncology.com/blog/ten- trends-transforming-the-business-of-oncology-2: OBR Oncology; 2011.
Abrams HR, Durbin S, Huang CX, Johnson SF, Nayak RK, Zahner GJ, et al. Financial toxicity in cancer care: origins, impact, and solutions. Transl Behav Med. 2021;11(11):2043-54.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer Statistics, 2008. CA Cancer J. Clin. 2008;58:71-96.
Morgan G, Ward R, Barton M. The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clinical Oncology. 2004;16:549-60.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57-70.
Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646-74.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;6:519-30.
Warburg O. On the origin of cancer cells. Science. 1956;123:309.
Wang Z, Jensen MA, Zenklusen JC. A Practical Guide to The Cancer Genome Atlas (TCGA). Methods Mol. Biol. 2016;1418:111-41.
Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578(7793):94-101.
Blum A, Wang P, Zenklusen JC. SnapShot: TCGA-Analyzed Tumors. Cell. 2018;173(2):530.
Christofferson T. T ripping over the truth. Charlston, SC: CreateSpace; 2014.
Watson J. To Fight Cancer, Know the Enemy. https://www.nvtimes.com/2009/08/06/opinion/06watson.html. Op-Ed Contribution ed: New York Times; 2009.
Szent-Gyorgyi A. The living state and cancer. Proc. Natl. Acad. Sci. U. S. A. 1977;74:2844-7.
Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutrition & Metabolism. 2010;7:7.
Seyfried TN. Cancer as a metabolic disease. On the origin, management, and prevenion of cancer. Hoboken, New Jersey: Wiley; 2012.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029-33.
Galluzzi L, Morselli E, Kepp O, Vitale I, Rigoni A, Vacchelli E, et al. Mitochondrial gateways to cancer. Molecular Aspects of Medicine. 2010;31:1-20.
John AP. Dysfunctional mitochondria, not oxygen insufficiency, cause cancer cells to produce inordinate amounts of lactic acid: the impact of this on the treatment of cancer. Medical Hypotheses. 2001;57:429-31.
Guezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaria G, Kim H, et al. The bioenergetic signature of cancer; a marker of tumor progression. Cancer Res. 2002;15:6674-81.
Kiebish MA, Han X, Cheng H, Chuang JH, Seyfried TN. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 2008;49:2545-56.
Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. PNAS. 2005;102:5992-7.
Chen Y, Cairns R, Papandreou I, Koong A, Denko NC. Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PloS ONE. 2009;4:e7033.
Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643-9.
Roskelley RC, Mayer N, Horwitt BN, Salter WT. Studies in cancer. VII. Enzyme deficiency in human and experimental cancer. J. Clin. Invest. 1943;22:743-51.
Nowell PC. Tumor progression: a brief historical perspective. Seminars in Cancer Biology. 2002;12:261-6.
Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000;21:497-503.
Delsite R, Kachhap S, Anbazhagan R, Gabrielson E, Singh KK. Nucelar genes involved in mitochondria-to-nuceus communication in breast cancer cells. Molecular Cancer. 2002;1:6.
Israel BA, schaeffer WI. Cytoplasmic suppression of malignancy. In Vitro Cell Dev. Biol. 1987;23:627-32.
Howell AN, Sagar R. Tumorigenicity and its suppression in cybrids of mouse and Chinese hamster cell lines. Proc. Natl. Acad. Sci. U. S. A. 1978;75:2358-62.
Singh KK, Kulawiec M, Still I, Desouki MM, Geradts J, Matsui SI. Inter-genomic cross talk between mitochondria and the nucleus plays an important role in tumorigenesis. Gene. 2005;354:140-6.
Li L, Connelly MC, Wetmore C, Curran T, Morgan JI. Mouse embryos cloned from brain tumors. 2003(2733):2736.
Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, Chin L, et al. Reprogramming of a melanoma genome by nuclear transplantation. Gene & Development. 2004;18:1875-85.
Koike K. Hepatitis B virus X gene is implicated in liver carcinogenesis. Cancer Letters. 2009;286:60-8.
D'Agostino DM, Bernardi P, Chieco-Bianchi L, Ciminale V. Mitochondria as functional targets of proteins coded by human tumor viruses. 94. 2005(87):142.
Clippinger AJ, Bouchard MJ. Hepatitis B virus Hbx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential. J. Virol. 2008;82:6798-811.
Costanzo M, De Giglio MAR, Roviello GN. Deciphering the Relationship between SARS-CoV-2 and Cancer. Int. J Mol. Sci. 2023;24(9).
Department of Defence; Pageshttps://www.health.mil/Military-Health-Topics/Health- Readiness/AFHSD/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database.
Goubran H, Stakiw J, Seghatchian J, Ragab G, Burnouf T. SARS-CoV-2 and cancer: the intriguing and informative cross-talk. Transfus. Apher. Sci. 2022;61(4):103488.
Clough E, Chean KT, Inigo J, Tubbesing KE, Chandra D, Chaves L. Mitochondrial dynamics in SARS-CoV-2 spike protein treated human microglia: Implications for neuro-COVID. Journal of Neuroimmune Pharmacology. 2021;16:770-84.
Diaz-Resendiz KJ, Benitez-Trinidad AB, Covantes-Rosales CE, Toledo-Ibarra GA. Loss of mitochondrial membrane potential in leucocytes as post-COVID-19 sequelae. J. Leukoc. Biol. 2022.
Medini H, Zirmman A, Mishmar D. Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis. iScience. 2021;24:103471.
Pliss A, Kuzmin AN, Prasad PN, Mahajan SD. Michochondrial dysfunction: A prelude to neuropathogenesis of SARS-C0V-2. ACS Chem. Neurosci. 2022;13:308-12.
Mortezaee K, Majidpoor J. CD8(+) T Cells in SARS-CoV-2 Induced Disease and Cancer-Clinical Perspectives. Front Immunol. 2022;13:864298.
Bhardwaj K, Liu P, Leibowitz JL, Kao CC. The coronavirus endoribonuclease Nsp15 interacts with retinoblastoma tumor suppressor protein. J Virol. 2012;86(8):4294-304.
Sheng Y, Laister RC, Lemak A, Wu B, Tai E, Duan S, et al. Molecular basis of Pirh2-mediated p53 ubiquitylation. Nat Struct Mol Biol. 2008;15(12):1334-42.
Tan X, Cai K, Li J, Yuan Z, Chen R, Xiao H, et al. Coronavirus subverts ER-phagy by hijacking FAM134B and ATL3 into p62 condensates to facilitate viral replication. Cell Rep. 2023;42(4):112286.
Seneff S, Nigh G, Kyriakopoulos AM, McCullough PA. Innate immune suppression by SARS-C-V-2 mRNA vaccinations: The role of G-quadruplexes, exosomes and microRNAs. Food & Chemical Toxicology. 2022;164:113008.
Musella M, Manic G, De Maria R, Vitale I, Sistigu A. Type-I-interferons in infection and cancer: Unanticipated dynamics with therapeutic implications. Oncoimmunology. 2017;6(5):e1314424.
Bustamante E, Pedersen PL. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc. Natl. Acad. Sci U. S. A. 1977;74(9):3735-9.
Ciscato F, Ferrone L, Masgras I, Laquatra C, Rasola A. Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters. Int. J Mol. Sci. 2021;22(9).
Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer's stygian link to the "Warburg Effect" and a pivotal target for effective therapy. Semin. Cancer Biol. 2009;19(1):17-24.
Patra KC, Hay N. Hexokinase 2 as oncotarget. Oncotarget. 2013;4(11):1862-3.
Dach J. Cracking Cancer toolkit: Using repurposed drugs for cancer treatment. 1st ed: Medical Muse Press; 2020.
Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899-908.
Liu S, Chen S, Zeng J. TGF-B signaling: A complex role in tumorigenesis. Molecular Medicine Reports. 2018;17:699-704.
Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461-73.
Nowell CS, Radtke F. Notch as a tumour suppressor. Nature Reviews Cancer. 2017;17:145-59.
Rascio F, Spadaccino F, Rocchetti MT, Castellano G, Stallone G, Netti GS, et al. The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers. 2021;13:3949.
Carballo GB, Honorato JR, de Lopes GPF, Spohr TCLS. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018;16(1):11.
Larsen AR, Bai RY, Chung JH, Borodovsky A, Rudin CM, Riggins GJ, et al. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol. Cancer. Ther. 2015;14:3-13.
Awad RM, De Vlaeminck Y, Maebe J, Goyvaerts C, Breckpot K. Turn back the TIMEe: Targeting tumor infiltrating myeloid cells to revert cancer progression. Front. Immunol. 2023;9:1977.
Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC, Mu J, et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023;12:11149 - 65.
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J Med. 2005;353(8):793-802.
Cole K, Al-Kadhimi Z, Talmadge JE. Role of myeloid-derived suppressor cells in tumor recurrence. Cancer and Metastasis Reviews. 2023.
Ma T, Renz BW, Ilmer M, Koch D, Yang Y, Werner J, et al. Myeloid-Derived Suppressor Cells in Solid Tumors. Cells. 2022;11(2).
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1ͱ, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781-90.
Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc. Biol. 2015;98(6):913-22.
Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70(15):6139-49.
Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells. 2020;9(3).
Gallego-Ortega D, Ledger A, Roden DL, Law AM, Magenau A, Kikhytyak Z, et al. ELF5 drives lung metastasis in luminal breast cancer through recruitment of Gr1+ CD11b+ myeloid-derived suppressor cells. PLoS Biol. 2015;13:e1002330.
Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65(8):3044-8.
Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123-31.
Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70(1):99-108.
Sharabi A, tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC. Regulatory T cells in the treatment of disease. Nature Reviews. 2018;17:823-44.
Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer. 2020;19(1):116.
Raffin C, Vo LT, Bluestone JA. T(reg) cell-based therapies: challenges and perspectives. Nat. Rev Immunol. 2020;20(3):158-72.
Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol. Oncol. 2022;15(1):61.
Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080-9.
Wu T, Wu X, Wang HY, Chen L. Immune contexture defined by single cell technology for prognosis prediction and immunotherapy guidance in cancer. Cancer Commun. (Lond). 2019;39(1):21.
Becht E, Giraldo NA, Dieu-Nosjean MC, SautÁ"s-Fridman C, Fridman WH. Cancer immune contexture and immunotherapy. Curr. Opin. Immunol. 2016;39:7-13.
Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol. Immunol. 2018;15(5):458-69.
Giraldo NA, Becht E, Remark R, Damotte D, SautÁ"s-Fridman C, Fridman WH. The immune contexture of primary and metastatic human tumours. Curr. Opin. Immunol. 2014;27:8-15.
Fridman WH, PagÁ"s F, SautÁ"s-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev Cancer. 2012;12(4):298-306.
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J Cancer. 2013;108(4):914-23.
Cassetta L, Pollard JW. Tumor-associated macrophages. Curr. Biol. 2020;30(6):R246-R8.
Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084.
Kumari N, Choi SH. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin. Cancer Res. 2022;41(1):68.
Heng Y, Zhu X, Lin H, Jingyu M, Ding X, Tao L, et al. CD206(+) tumor-associated macrophages interact with CD4(+) tumor-infiltrating lymphocytes and predict adverse patient outcome in human laryngeal squamous cell carcinoma. J Transl. Med. 2023;21(1):167.
Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat. Rev Immunol. 2011;11(11):723-37.
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:12150.
Beury DW, Parker KH, Nyandjo M, Sinha P, Carter KA, Ostrand-Rosenberg S. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J Leukoc. Biol. 2014;96(6):1109-18.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev Immunol. 2021;21(8):485-98.
Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105(1):1-8.
Liu W, Wang W, Wang X, Xu C, Zhang N, Di W. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett. 2020;472:59-69.
Li X, Liu R, Su X, Pan Y, Han X, Shao C, et al. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol. Cancer. 2019;18(1):177.
Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462-72.
Zhao X, Qu J, Sun Y, Wang J, Liu x, Wang F, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8(18):30576- 86.
Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2017;147(1):181-7.
Komohara Y, Niino D, Ohnishi K, Ohshima K, Takeya M. Role of tumor-associated macrophages in hematological malignancies. Pathol. Int. 2015;65(4):170-6.
Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br. J Cancer. 2018;118(2):171-80.
D'Errico G, Alonso-Nocelo M, Vallespinos M, Hermann PC, AlcalÂj S, GarcÂ-a CP, et al. Tumor- associated macrophage-secreted 14-3-3 signals via AXL to promote pancreatic cancer chemoresistance. Oncogene. 2019;38(27):5469-85.
Gyori D, Lim EL, Grant FM, Spensberger D, Roychoudhuri R, Shuttleworth SJ, et al. Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. JCI Insight. 2018;3(11).
Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013;18:43- 73.
Fan CS, Chen LL, Hsu TA, Chen CC, Chua KV, Li CP, et al. Endothelial-mesenchymal transition harnesses HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal adenocarcinoma. J Hematol. Oncol. 2019;12(1):138.
Wang W, Liu Y, Guo J, He H, Mi X, Chen C, et al. miR-100 maintains phenotype of tumor- associated macrophages by targeting mTOR to promote tumor metastasis via Stat5a/IL-1ra pathway in mouse breast cancer. Oncogenesis. 2018;7(12):97.
Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human Tumor- Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell. 2019;35(4):588-602.
Debebe A, Medina V, Chen CY, Mahajan IM, Jia C, Fu D, et al. Wnt/b-catenin activation and macrophage induction during liver cancer development following steatosis. Oncogene. 2017;36(43):6020-9.
Chen Q, Zhang XH, Massagu J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20(4):538-49.
Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan J, et al. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF- b signaling pathway. J Exp Clin. Cancer Res. 2019;38(1):310.
Klimp AH, Hollema H, Kempinga C, van der Zee AG, de Vries EG, Daemen T. Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor- associated macrophages. Cancer Res. 2001;61(19):7305-9.
Pan B, Ge L, Xun YQ, Chen YJ, Gao CY, Han X, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int. J Behav. Nutr. Phys. Act. 2018;15(1):72.
Majety M, Runza V, Lehmann C, Hoves S, Ries CH. A drug development perspective on targeting tumor-associated myeloid cells. FEBS J. 2018;285(4):763-76.
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576- 90.
Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc. Natl. Acad. Sci U. S. A. 2014;111(30):E3053-E61.
McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96(5):1789-97.
Heinmoller E, Weinel RJ, Heidtmann HH, Salge U, Seitz R, Schmitz I, et al. Studies on tumor-cell- induced platelet aggregation in human lung cancer cell lines. J Cancer Res Clin. Oncol. 1996;122(12):735-44.
Grignani G, Pacchiarini L, Ricetti MM, Dionigi P, Jemos V, Zucchella M, et al. Mechanisms of platelet activation by cultured human cancer cells and cells freshly isolated from tumor tissues. Invasion Metastasis. 1989;9(5):298-309.
Nassar D, Blanpain C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev Pathol. 2016;11:47-76.
Huang Z, Wu T, Liu AY, Ouyang G. Differentiation and transdifferentiation potentials of cancer stem cells. Oncotarget. 2015;6(37):39550-63.
Singh VK, Saini A, Chandra R. The Implications and Future Perspectives of Nanomedicine for Cancer Stem Cell Targeted Therapies. Front Mol. Biosci. 2017;4:52.
Dionisio MR, Vieira AF, Carvalho R, Conde I, Oliveira M, Gomes M, et al. BR-BCSC Signature: The Cancer Stem Cell Profile Enriched in Brain Metastases that Predicts a Worse Prognosis in Lymph Node-Positive Breast Cancer. Cells. 2020;9(11).
Kurtova AV, Xiao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517(7533):209- 13.
Reiter RJ, Rosales-Corral SA, TTan DX, Acuna-Castroviejo D, Qin L, Yang SF, et al. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017;18:843.
Fong D, Christensen CT, Chan MM. Targeting Cancer Stem Cells with Repurposed Drugs to Improve Current Therapies. Recent Pat Anticancer Drug Discov. 2021;16(2):136-60.
Proietti S, Cucina A, D'Anselmi F, Dinicola S, Pasqualato A, Lisi E, et al. Melatonin and vitamin d 3 synergistically down-regulate Akt and MDM2 leading to TGFÎ2-1-dependent growth inhibition of breast cancer cells. J Pineal Res. 2011;50(2):150-8.
Dominguez-Gomez G, Chavez-Blanco A, Medina-Franco JL, Saldivar-Gonzalez F, Flores- Torrontegui Y, Juarez M, et al. Ivermectin as an inhibitor of cancer stem-like cells. Mol. Med Rep. 2018;17(2):3397-403.
Puar YR, Shanmugam MK, Fan L, Arfuso F, Sethi G, Tergaonkar V. Evidence for the Involvement of the Master Transcription Factor NF-ΰB in Cancer Initiation and Progression. Biomedicines. 2018;6(3).
Farvid MS, Sidahmed E, Spence ND, Mante AK, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2021;36(9):937-51.
Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, et al. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose-Response Meta-Analysis. Nutrients. 2019;11(4).
Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW. Effect of vitamin d supplementation, omega- 3 fatty acid supplementation, or a strengh-training excercise program on clinical outcomes in older adults. the DO-HEALTH randomized clinical trial. JAMA. 2020;324:1855-68.
Bischoff-Ferrari HA, Willett WC, Manson JE, Dawson-Hughes B, Manz MG, Theller R, et al. Combined vitamin d , omega-3 fatty acids, and a simple home exercise program may reduce cancer risk among active adults aged 70 and older: A randomized clinical trial. Front. Aging. 2022;3:852643.
Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. vitamin d Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J Med. 2019;380(1):33-44.
Li XX, Liu C, Dong SL, Ou CS, Lu JL, Ye JH. Anticarcinogenic potentials of tea catechins. Front. Nutr. 2022;9:1060783.
Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011;82(12):1807-21.
Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes. Metab. 2014;16(11):1165-73.
Tseng CH. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer. 2014;50(16):2831-7.
Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res (Phila). 2014;7(9):867-85.
Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37(3):207-18.
Kim TL, Jeong GH, Yang JW, Lee KH, Kronbichler A, van der Vliet HJ, et al. Tea Consumption and Risk of Cancer: An Umbrella Review and Meta-Analysis of Observational Studies. Adv Nutr. 2020;11(6):1437-52.
Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, et al. Safety of green tea extracts. A systematic review by the US Pharmacopeia. Drug Safety. 2008;31:464-84.
Talib WH, Alsayed AR, Abuawad A, Daoud S, Mahmod AI. Melatonin in cancer treatment: Current knowledge and future opportunities. Molecules. 2021;26:2506.
Williamson T, Bai RY, Staedtke V, Huso D, Riggins GJ. Mebendazole and a non-steroidal antiinflammatory combine to reduce tumor initiation in a colon cancer preclinical model. Oncotarget. 2016;7:68571-84.
Huang W, Sundquist J, Sundquist K, Ji J. Use of Phosphodiesterase 5 Inhibitors Is Associated With Lower Risk of Colorectal Cancer in Men With Benign Colorectal Neoplasms. Gastroenterology. 2019;157(3):672-81.
Agrawal S, Vamadevan P, Mazibuko N, Bannister R, Swery R, Wilson S. A new method for ethical and efficient evidence generation for off-label medication use in oncology (A case study in glioblastoma). Front. Pharmacol. 2019;10:681.
McLelland J. How to starve cancer... and then kill it with ferroptosis. 2nd Edition ed. Central Books, United Kingdom: Agenor Publishing; 2021.
Mukherjee P, Sotnokov AV, Mangian HJ, Zhou JR, Visek WJ, Clinton SK. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J. Natl. Cancer Inst. 1999;91:512-23.
Mavropoulos JC, Buschemeyer WC, Tewari AK, Rokheld D, Pollak M, Zhao Y. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCap prostate coancer Zenograft model. Cancer Prev. Pre. 2009;2:557-65.
Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learnt form 30 years of calorie restriction research. Carcinogenesis. 2010;31:83-9.
Kari FW, Dunn SE, French JE, Barrett JC. Roles for insulin-like growth factor-1 in mediating the anti-carcinogenic effects of caloric restriction. J. Nutr. Health Aging. 1999;3:92-101.
Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grambsch PL, Grande JP, et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr. Cancer. 2009;61:265-75.
Thompson HJ, Jiang W, Zhu Z. Mechanisms by which energy restriction inhibits carcinogenesis. Adv. Exp. Med. Biol. 1999;470:77-84.
Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutrition & Metabolism. 2007;4:5.
McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63:286-91.
Meynet O, Ricci JE. Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends in Molecular Medicine. 2014;20:419-27.
Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies to fuel metabolism, signaling, and therapeutics. Cell Metabolism. 2017;25:262-84.
Hwang CY, Choe W, Yoon KS, Ha J, Kim SS, Yeo EJ, et al. Molecular mechanisms for ketone body metabolism, signaling functions, and therapeutic potential in cancer. Nutrients. 2022;14:4932.
Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends in Endocrinology and Metabolism. 2014;25:42-52.
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by B-hydroxybutyrate, and endogenous histone deacetylase inhibitor. Science. 2013;339:211-4.
Mulrooney TJ, Marsh J, Urits I, Seyfried TN, Mukherjee P. Influence of caloric restriction on constitutive expression of NFkB in an experimental mouse astrocytoma. PloS ONE. 2011;6(3):e18085.
Chi JT, Lin PH, Tolstikov V, Howard L, Chen EY, Bussberg V, et al. Serum metabolomic analysis of men on a low-carbohydrate diet for biochemically recurrent prostate cancer reveals the potential role of ketogenesis to slow tumor growth: a secondary analysis of the CAPS2 diet trial. Prostate Cancer Prostatic. Dis. 2022;25(4):770-7.
Evangeliou AE, Spilioti MG, Vassilakou D, Goutsaridou F, Seyfried TN. Restricted Ketogenic Diet Therapy for Primary Lung Cancer With Metastasis to the Brain: A Case Report. Cureus. 2022;14(8):e27603.
Seyfried TN, Shivane AG, Kalamian M, Maroon JC, Mukherjee P, Zuccoli G. Ketogenic Metabolic Therapy, Without Chemo or Radiation, for the Long-Term Management of IDH1-Mutant Glioblastoma: An 80-Month Follow-Up Case Report. Front Nutr. 2021;8:682243.
Meidenbauer JJ, Mukherjee P, Seyfried TN. The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr. Metab (Lond). 2015;12:12.
Miyata Y, Shida Y, Hakariya T, Sakai H. Anti-cancer effects of green tea polyphenols against prostate cancer. Molecules. 2019;24:193.
Yang C, Sudderth J, Dang T, Bachoo RG, McDonald JG, Deberardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairements of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986-93.
Li M, Li C, Allen A, Stanley CA, Smith TJ. The structure and allosteric regulaion of mammalian glutamate dehydrogenase. Arch. Biochem. Biophys. 2012;519:69-80.
Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, et al. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J. Biol. Chem. 2006;281:10214-21.
Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234-40.
Ifland J, Marcus MT, Preuss HG. Processed Food Addiction. Foundations, Assessment, and Recovery. Boca Rotan, FL: CRC Press; 2018.
Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69(10):1867-76.
Lee KA, Luong MK, Shaw H, Nathan P, Bataille V, Spector TD. The gut microbiome: what the oncologist ought to know. Br J Cancer. 2021;125(9):1197-209.
Sadrekarimi H, Gardanova ZR, Bakhshesh M, Ebrahimzadeh F, Yaseri AF, Thangavelu L, et al. Emerging role of human microbiome in cancer development and response to therapy: special focus on intestinal microflora. J Transl Med. 2022;20(1):301.
Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7(271):271ps1.
Boursi B, Mamtani R, Haynes K, Yang YX. Recurrent antibiotic exposure may promote cancer formation--Another step in understanding the role of the human microbiota? Eur J Cancer. 2015;51(17):2655-64.
Cao Y, Wu K, Mehta R, Drew DA, Song M, Lochhead P, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672-8.
Banting W. Letter on Corpulence, Addressed to the Public. 3rd ed. London, UK: Harrison; 1864.
Creed SA. The Real Meal Revolution. The Radical, Sustainable Approach to Healthy Eating. London, UK: Robinson; 2015.
Meadows W. The Banting Diet: Letter on Corpulence: FCD Publising; 2015.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105.
Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer Cachexia: Its Mechanism and Clinical Significance. Int J Mol Sci. 2021;22(16).
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489-95.
Baldwin C, Spiro A, McGough C, Norman AR, Gillbanks A, Thomas K, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet. 2011;24(5):431-40.
Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, Germain C, Blanc JF, Dauba J, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS One. 2014;9(9):e108687.
Advani SM, Advani PG, VonVille HM, Jafri SH. Pharmacological management of cachexia in adult cancer patients: a systematic review of clinical trials. BMC Cancer. 2018;18(1):1174.
Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17(4):519-31.
Antoni R, Johnston KL, Collins AL, Robertson MD. Effects of intermittent fasting on glucose and lipid metabolism. Proc. Nutr. Soc. 2017;76(3):361-8.
Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosppression. Cell Stem Cell. 2014;14:810-23.
de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019;381:2541-51.
Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017;39:46-58.
Vasim I, Majeed CN, DeBoer MD. Intermittent fasting and metabolic health. Nutrients. 2022;14:631.
Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell. Biol. 1992;119:301-11.
Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS. 1993;333:169-74.
Antures F, Erustes AG, Costa AJ, Nascimento AC, Bincoletto C, Ureshino RP, et al. Autophagy and intermittent fasting: the connection for cancer therapy? Clinics. 2018;73 (Suppl 1):e814S.
Fung J, Moore J. The complete guide to fasting: Victory Belt Publishing; 2016.
Morishita H, Mizushima N. Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol. 2019;35:3.1-3.23.
Munoz A, Grant WB. vitamin d and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients. 2022;14(7).
Das M, Ellies LG, Kumar D, Sauceda C, Oberg A, Gross E, et al. Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nature Communications. 2021;12:565.
Buschemeyer WC, Klink JC, Mavropoulos JC, Poulton SH, Hursting SD. Effectof intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate. 2010;70:1037-43.
Sundaram S, Yan L. Time-restricted feeding mitigates high fat diet enhanced mammary tumorigenesis in MMTV-PyMT mice. Nutrition Research. 2018;59:72-9.
Yan L, Sundaram S, Mehus AA, Picklo MJ. Time-restricted feeding attenuates high-fat diet- enhanced spontaneous metastasis of Lewis lung carcinoma in mice. Anticancer Research. 2019;39:1739-48.
Sun P, Wang H, He Z, Chen X, Wu Q, Chen W, et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-assocaited macrophages. Oncotarget. 2017;8:74649-60.
Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Pistoria V, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Science Translational Medicine. 2012;4:124ra27.
Xu R, Ji Z, Xu C, Zhu J. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers. A systematic review and meta-analysis. Medicine. 2018;97:46.
Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2006;144:337-43.
Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC, et al. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. The Oncologist. 2014;19:637-8.
Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: Recent advances and future directions. Cancer Discov. 2019;9:1167-81.
Zeh HJ, Bahary N, Boone BA, Singh AD, Normolle Dp, Hogg ME. A randomized Phase II preoperative study of autophagy inhibition with high-dose hydroxychloroquine and Gemcitabine/Nab-Paclitaxel in pancreatic cancer patients. Clinical Cancer Research. 2020;26:3126-34.
Agrawal S, Wozniak M, Luc M, Makuch S, Pielka E, Agrawal AK, et al. Insulin enhancement of the antitumor activity of chemotherapeutic agents in colorectal cancer is linked with downregulating PIK3CA and GRB2. Sci Rep. 2019;9(1):16647.
Sissung TM, Schmidt KT, Figg WD. Insulin potentiation therapy for cancer? Lancet Oncol. 2019;20(2):191-2.
Ayre SG, Bellon DP, Garcia DP, Jr. Insulin, chemotherapy, and the mechanisms of malignancy: the design and the demise of cancer. Med Hypotheses. 2000;55(4):330-4.
Lasalvia-Prisco E, Cucchi S, VÂjzquez J, Lasalvia-Galante E, Golomar W, Gordon W. Insulin- induced enhancement of antitumoral response to methotrexate in breast cancer patients. Cancer Chemother. Pharmacol. 2004;53(3):220-4.
Bernstein J. MIA In the War on Cancer: Where are the Low-Cost Treatments. https://www.propublica.org/article/where-are-the-low-cost-cancer-treatments: ProPublica; 2014.
Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database of Syst. Rev. 2014;4:MR000034.
Pantziarka P, Verbaanderd C, Sukhatme V, Rica C, I, Crispino S, Gyawali B, et al. ReDO_DB: the repurposing drugs in oncology database. Ecancermedicalscience. 2018;12:886.
Campos-Carrillo A, Weitzel JN, Sahoo P, Rockne R, Mokhnatkin JV, Murtaza M, et al. Circulating tumor DNA as an early cancer detection tool. Pharmacol. Ther. 2020;207:107458.
Moding EJ, Nabet BY, Alizadeh AA, Diehn M. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov. 2021;11(12):2968-86.
Ambrosone CB, Zirpoli GR, Hutson AD, McCann WE, McCann SE, Barlow WE, et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J Clin. Oncol. 2020;38(8):804-14.
Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl. Cancer Inst. 2008;100(11):773-83.
Marik PE. Hydrocortisone, Ascorbic Acid and Thiamine (HAT therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients. 2018;10:1762.
Cazzaniga ME, Cordani N, Capici S, Cogliati V, Riva F, Cerrito MG. Metronomic Chemotherapy. Cancers (Basel). 2021;13(9).
Wichmann V, Eigeliene N, Saarenheimo J, Jekunen A. Recent clinical evidence on metronomic dosing in controlled clinical trials: a systematic literature review. Acta Oncol. 2020;59(7):775-85.
Liu Y, Gu F, Liang J, Dai X, Wan C, Hong X, et al. The efficacy and toxicity profile of metronomic chemotherapy for metastatic breast cancer: A meta-analysis. PloS ONE. 2017;12(3):e0173693.
Barclay AW, Augustin LS, Brighenti F, Delport E, Henry CJ, Sievenpiper JL, et al. Dietary glycaemic index labelling: A global perspective. Nutrients. 2021;13:3244.
Matthan NR, Ausman LM, Meng H, Tighiouart H, Lichtenstein AH. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am. J. Clin. Nutr. 2016;104:1004-13.
Inchauspe J. Glucose Revolution. New York: Simon & Schuster; 2022.
Santos HO, de Moraes WM, da Silva GA, restes J, Schoenfeld BJ. Vinegar (acetic acid) intake on glucose metabolism: A narrative review. Clinical Nutrition ESPEN. 2019;32:1-7.
Shishehbor F, Mansoori A, Shirani F. Vinegar consumption can attenuate postproandial glucose and insulin responses: a systematic review and meta-analysis of clinical trials. Diabetes Research and Clinical Practice. 2017;127:1-9.
Siddiqui FJ, Assam PN, de Souza NN, Sultana R, Dalan r, Chan ES. Diabetes control: Is vinegar a promising candidate to help achieve targets?? Journal of Evidence-Based Integrative Medicine. 2018;23:1-12.
Petsiou EI, Mitrou PI, Raptis SA, Dimitriadis GD. Effect and mechanisms of action of vinegar on glucose metabolism,lipid profile, and body weight. Nutrition Reviews. 2014;72:651-61.
Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, et al. Low-volume high- intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. Journal of Applied Physiology. 2011;111(6):1554-60.
Praet SF, Manders RJ, Lieverse AG, Kuipers H, Stehouwer CD, Keizer HA. Influence of acute excercise on hyperglycemia in insulin-treated type-2 diabets. Medicine & Science in Sports & Exercise. 2006(2037):2044.
Dipla K, Zafeiridis A, Mintziori G, Boutou AK, Goulis DG, Hackney AC. Exercise as a therapeutic intervention in gestational diabetes mellitus. Endocrines. 2021;2:65-78.
Halilton MT, Hamilton D, Zderic TW. A potent physiological method to magnify and sustain soleus oxidative metabolism improves glucose and lipid regulation. iScience. 2022;25:104869.
Yu EW, Gao L, Stastka P, Cheney MC, Soto MT, Ford CB, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PloS ONE. 2020;17:e1003051.
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376-81.
Sung MM, Kim TT, Denou E, Soltys CL, Hamza SM, Byrne NJ, et al. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes. 2017;66:418-25.
Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146:1525-33.
Rebello CJ, Burton J, Heiman M, Greenway FL. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilto trial. J. Diabetes Complications. 2015;29:1272-6.
Maruvada P, Leone V, Kaplan LM, Chang EB. The human microbiome and obesity: Moving beyond associations. Cell Host & Microbe. 2017;22:589-99.
Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: Where are we now? Diabetes Research and Clinical Practice. 2018;146:111-8.
Teicholz N. The Big FAT Suprise. Why butter, meat and cheese belong in a healthy diet. New York: Simon & Schuster; 2014.
Teicholz N. A short history of saturated fat: the making and unmaking of a scientific consensus. Curr. Opin. Endo. Diab. Obesity. 2023;30:65-71.
Astrup A, Teicholz N, Magkos F, Bier DM, Brenna JT, King JC, et al. Dietary saturated fats and health: Are the U.S. Guidelines evidence-based? Nutrients. 2021;13:3305.
Keys A, Mienotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986;124:903-15.
Page IH, Allen EV, Chamberlain FL, Keys A, Stamler J, Stare FJ. Dietary fat and its relation to heart attacks and strokes. Circulation. 1961;23:133-6.
Dayton S, Pearce ML, Hashimoto S, Fakler LJ, Hiscock E, Dixon WJ. A controlled clinical trial of a diet high in unsaturated fat. Preliminary observations. N. Engl. J. Med. 1962;266:1017-23.
Ramsden CE, Zamora D, Faurot KR, Broste SK, Frantz RP, Davis JM, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data form the Minnesota Coronary Experiment (1968-1973). BMJ. 2016;353:i1246.
Ramsden CE, Zamora D, Faurot KR, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevenion of heart disease and death: evaluation of recovered data form the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013;346:e8707.
Caccamo AE, Scaltriti M, Caporali A, D'Arca D, Scorcioni F, Astancolle S, et al. Cell detachment and apoptosis induction of immortalized prostate epithelial cells are associated with early accumulation of a 45 kDa nuclear isoform of clusterin. Biochem. J. 2004;382:157-68.
Scaltriti M, Santamaria A, Paciucci R, Bettuzzi S. Intracellular clusterin induces G2-M phase arrest and cell death in PC-3 prostate cancer cells. Cancer Research. 2004;64:6174-82.
Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Letters. 1995;96:239-43.
El-Nashar HA, Aly SH, Ahmadi A, El-Shazly M. The impact of polyphenolics in the management of breast cancer: Mechanistic aspects and recent patents. Recent Patents on Anti-Cancer Drug Discovery. 2022;17:358-79.
Kubatka P, Mazurakova A, Samec M, Koklesova L, Zhai K, Kajo K, et al. Flavonoids against non- physiologic inflammation attributed to cancer initiation, development, and progression - 3PM pathways. EPMA Journal. 2021;12:559-87.
Katiyar S, Mukhtar H. Tea in chemoprevention. International Journal of Oncology. 1996;8:221- 38.
Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402:685-9.
Rashidi B, Malekzadeh M, Goodarzi M, Masoudifar A, Mirzaei H. Green tea and its antiangiogenesis effects. Biomed Pharmacother. 2017;89:949-56.
Yoon JW, Lee JS, Kim BM, Ahn J, Yang KM. Catechin-7-O-xyloside induces apoptosis via endoplasmic reticulum stress and mitochondrial dysfunction in human non-small cell lung carcinoma H1299 cells. Oncology Reports. 2014;31:314-20.
Sun H, Yin M, Hao D, Shen Y. Anti-cancer activity of catechin against A549 lung carcinoma cells by inducion of cyclin kinase inhibitor p21 and suppression of cyclin E1 and p-AKT. Appl. Sci. 2020;10:2065.
Song Q, Zhang G, Wang B, Cao G, Li D, Wang Y, et al. Reinforcing the combinational immuno- oncotherapy of switching "cold" tumor to "hot" by responsive penetrating nanogels. ACS Appl. Mater. Interface. 2021;13:36824-38.
Menon DR, Li Y, Yamauchi T, Osborne DG, Vaddi PK, wempe MF, et al. EGCG inhibits tumor growth in melanoma by targeting JAK-STAT signaling andits downstream PD-L1/PD-L2-PD1 axis in tumors and enhancing cytotoxic T-cell responses. Pharmaceuticals. 2021;14:1081.
McCarty MF, LLoki-Assanga S, Lujany LM. Nutraceutical targeting of TLR4 signaling has potential for prevention of cancer cachexia. Medical Hypotheses. 2019;132:109326.
Mukherjee S, Hussaini R, White R, Atwi D, Fried A, Sampat S, et al. TriCurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to casue suppression of HPV+ tumors. Cancer Immunology Immunotherapy. 2018;67:761-74.
Xu P, Yan F, Zhao Y, Chen X, Sun S, Wang Y, et al. Green tea polyphenol EGCG attenuates MDSCs- mediated immunosuppresion through canonical and non-canoical pathways in a 4T1 murine breast cancer model. Nutrients. 2020;12:1042.
McCarty MF, Iloki-Assanga S, Lujany LML. Nutraceutical targeting of TLR4 signaling has potential for prevention of cancer cachexia. Med Hypotheses. 2019;132:109326.
Rogovskii VS, Popov SV, Sturov NV, Shimanovski NL. The possibility of preventive and therapeutic use of green tea catechins in prostate cancer. Anti-Cancer Agents in Medicinal Chemistry. 2019;19:1223-31.
Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Arch. Toxicol. 2015;89:1175-91.
Colunga Biancatelli RM, Berrill M, Mohammed YH, Marik PE. Melatonin for the treatment of sepsis: the scientific rationale. J. Thorac. Dis. 2020;12 (Suppl 1):S54-S65.
Jung B, Ahmad N. Melatonin in cancer management: Progress and promise. Cancer Res. 2006;66:9789-93.
Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, et al. Update on melatonin receptors: IUPHAR Review 20. Br. J Pharmacol. 2016;173(18):2702-25.
Yeager RL, Oleske DA, Sanders RA, Eells JT, Henshel DS. Melatonin as a principal component of red light therapy. Medical Hypotheses. 2007;69:372-6.
Tan DX, Reiter RJ, Zimmerman S, Hardeland R. Melatonin: Both a messenger of darkness and a participant in cellular actions of non-visible solar radiation of near infrared light. Biology. 2023;12:89.
Manouchehri E, Taghipour A, Ghavami V, Ebadi A, Homaei F, Latifnejad RR. Night-shift work duration and breast cancer risk: an updated systematic review and meta-analysis. BMC Womens Health. 2021;21(1):89.
Wise J. Danish night shift workers with breast cancer awarded compensation. BMJ. 2009;338:b1152.
Mortezaee K, Najafi M, Farhood B, Ahmadi A, Potes Y, Shabeeb D, et al. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 2019;228:228-41.
Akbarzadeh M, Movassaghpour AA, Ghanbari H, Kheirandish M, Fathi MN, Rahbarghazi R, et al. The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells. Sci Rep. 2017;7(1):17062.
Reiter RJ, Sharma R, Ma Q, Rosales-Corral SA, Escames G. Inhibition of mitochondrial pyruvate dehydrogenase kinase: a proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2019;2:105-19.
Sanchez-Sanchez AM, AAntolin I, Puente-Moncada N, Suarez S, Rodriguez C. Melatonin cytotoxicity is associated to Warburg effect inhibition in Ewing sarcoma cells. PloS ONE. 2015;10:e0135420.
Hevia D, Gonzalez-Menendez P, Fernandez-Fernandez M, Cueto S, Rodriguez-Gonzalez P, Garcia- Alonso JI, et al. Melatonin Decreases Glucose Metabolism in Prostate Cancer Cells: A (13)C Stable Isotope-Resolved Metabolomic Study. Int. J Mol. Sci. 2017;18(8).
Perfilyeva YV, Ostapchuk YO, Abdolla N, Tleulieva R, Krasnoshtanov VC, Belyaev NN. Exogenous Melatonin Up-Regulates Expression of CD62L by Lymphocytes in Aged Mice under Inflammatory and Non-Inflammatory Conditions. Immunol. Invest. 2019;48(6):632-43.
Liu H, Xu L, Wei JE, Xie MR, Wang SE, Zhou RX. Role of CD4+ CD25+ regulatory T cells in melatonin-mediated inhibition of murine gastric cancer cell growth in vivo and in vitro. Anat. Rec. 2011;294(5):781-8.
Seely D, Wu P, Fritz H, Kennedy DA, Tsui T, Seely AJ. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integrative Cancer Therapies. 2012;11:293-303.
Holick MF. vitamin d deficiency. N. Engl. J. Med. 2002;357:266-81.
Brandi ML. Indications on the use of vitamin d and vitamin d metabolites in clinical phenotypes. Clin. Cases. Miner. Bone Metab. 2010;7(3):243-50.
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin d insufficiency in an adult normal population. Osteoporos. Int. 1997;7(5):439-43.
Vieth R. Why the optimal requirement for vitamin d 3 is probably much higher than what is officially recommended for adults. J Steroid Biochem. Mol. Biol. 2004;89-90(1-5):575-9.
Wimalawansa SJ. Rapidly increasing serum 25(OD)D boosts immune system, against infections - Sepsis and COVID-19. Nutrients. 2022;14:2997.
Wimalawansa SJ. Effective and practical ways to overcome vitamin d deficiency. J. Family Med. Community Health. 2021;8:1-8.
Reddy P, Edwards LR. Magnesium supplementation in vitamin d deficiency. Am. J. Ther. 2019;26:e124-e32.
Schwalfenberg GK. Vitamins K1 and K2: The emerging group of vitamins required for human health. Journal of Nutrition and Metabolism. 2017;2017:6254836.
Duan F, Mei C, Yang L, Zheng J, Lu H, Xia Y, et al. Vitamin K2 promotes PI3K/AKT/HIF-1a- mediated glycolysis that leads to AMPK-dependent autophagic cell death in bladder cancer cells. Sci Rep. 2020;10(1):7714.
Tokita H, Tsuchida A, Miyazawa K, Ohyashiki K, Katayanagi S, Sudo H, et al. Vitamin K2-induced antitumor effects via cell-cycle arrest and apoptosis in gastric cancer cell lines. Int. J Mol. Med. 2006;17(2):235-43.
Welsh J, Bak MJ, Narvaez CJ. New insights into vitamin K biology with relevance to cancer. Trends Mol. Med. 2022;28(10):864-81.
Nimptsch K, Rohrmann S, Kaaks R, Linseisen J. Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am. J Clin. Nutr. 2010;91(5):1348-58.
Carlberg C, Velleuer E. vitamin d and the risk for cancer: A molecular analysis. Biochem. Pharmacol. 2022;196:114735.
Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. vitamin d : modulator of the immune system. Current Opinion in Pharmacology. 2010;10(4):482-96.
Bartley J. vitamin d : emerging roles in infection and immunity. Expert Review of Antiinfective Therapy. 2010;8(12):1359-69.
Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, et al. vitamin d and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903.
Ng K, Venook AP, Sato K, Yuan C, Hollis BW, Chang IW, et al. vitamin d status and survival of metastatic colorectal cancer patients: Results form CALGB/SWOG 80405 (Alliance) [Abstract]. J. Clin. Oncol. 2015;33:3503.
Sha S, Nguyen TMN, Kuznia S, Niedermaier T, Zhu A, Brenner H, et al. Real-world evidence for the effectiveness of vitamin d supplementation in reduction of total and cause-specific mortality. J Intern Med. 2023;293(3):384-97.
Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. vitamin d supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2014(1):CD007470.
Hossain S, Beydoun MA, Beydoun HA, Chen X, Zonderman AB, Wood RJ. vitamin d and breast cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. ESPEN. 2019;30:170-84.
Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin d supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673.
Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM, et al. Effect of High-Dose vs Standard-Dose vitamin d 3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA. 2019;321(14):1370-9.
Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin d 3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60(8):2304-12.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin d in reducing cancer risk and progression. Nat. Rev Cancer. 2014;14(5):342-57.
Mathieu C, Adorini L. The coming of age of 1,25-dihydroxy vitamin d (3) analogs as immunomodulatory agents. Trends Mol. Med. 2002;8(4):174-9.
Zheng W, Cao L, Ouyang L, Zhang Q, Duan B, Zhou W, et al. Anticancer activity of 1,25- (OH)(2)D(3) against human breast cancer cell lines by targeting Ras/MEK/ERK pathway. Onco. Targets Ther. 2019;12:721-32.
Abu El Maaty MA, Wolfl S. Effects of 1,25(OH)2 D3 on Cancer Cells and Potential Applications in Combination with Established and Putative Anti-Cancer Agents. Nutrients. 2017;9(1).
Yang ES, Burnstein KL. vitamin d inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem. 2003;278(47):46862-8.
Krishnan AV, Swami S, Feldman D. vitamin d and breast cancer: inhibition of estrogen synthesis and signaling. J Steroid Biochem. Mol. Biol. 2010;121(1-2):343-8.
Palmer HG, Sanchez-Carbayo M, Ordonez-Moran P, Larriba MJ, Cordonn-Cardo C, Munoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxy vitamin d 3 in human colon cancer cells. Cancer Res. 2003;63(22):7799-806.
Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. vitamin d (3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154(2):369-87.
Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65(17):7917-25.
Larriba MJ, Garcia de Herreros A, Munoz A. vitamin d and the Epithelial to Mesenchymal Transition. Stem Cells Int. 2016;2016:6213872.
Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL. Antiproliferative effects of 1 alpha,25- dihydroxy vitamin d (3) and vitamin d analogs on tumor-derived endothelial cells. Endocrinology. 2002;143(7):2508-14.
Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, et al. Circulating 25- hydroxy vitamin d levels and survival in patients with colorectal cancer. J Clin. Oncol. 2008;26(18):2984-91.
Johansson H, Spadola G, Tosti G, MandalÄ M, Minisini AM, Queirolo P, et al. vitamin d Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial. Nutrients. 2021;13(6).
Yuan C, Sato K, Hollis BW, Zhang S, Niedzwiecki D, Ou FS, et al. Plasma 25-Hydroxy vitamin d Levels and Survival in Patients with Advanced or Metastatic Colorectal Cancer: Findings from CALGB/SWOG 80405 (Alliance). Clin. Cancer Res. 2019;25(24):7497-505.
Mezawa H, Sugiura T, Watanabe M, Norizoe C, Takahashi D, Shimojima A, et al. Serum vitamin d levels and survival of patients with colorectal cancer: post-hoc analysis of a prospective cohort study. BMC Cancer. 2010;10:347.
Zgaga L, Theodoratou E, Farrington SM, Din FV, Ooi LY, Glodzik D, et al. Plasma vitamin d concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin. Oncol. 2014;32(23):2430-9.
Toriola AT, Nguyen N, Scheitler-Ring K, Colditz GA. Circulating 25-hydroxy vitamin d levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol. Biomarkers Prev. 2014;23(6):917-33.
Tretli S, Schwartz GG, Torjesen PA, Robsahm TE. Serum levels of 25-hydroxy vitamin d and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population- based study. Cancer Causes Control. 2012;23(2):363-70.
Robsahm TE, Schwartz GG, Tretli S. The Inverse Relationship between 25-Hydroxy vitamin d and Cancer Survival: Discussion of Causation. Cancers (Basel). 2013;5(4):1439-55.
Chen QY, Kim S, Lee B, Jeong G, Lee DH, Keum N, et al. Post-Diagnosis vitamin d Supplement Use and Survival among Cancer Patients: A Meta-Analysis. Nutrients. 2022;14(16).
Vaughan-Shaw PG, Buijs LF, Blackmur JP, Theodoratou E, Zgaga L, Din FVN, et al. The effect of vitamin d supplementation on survival in patients with colorectal cancer: systematic review and meta-analysis of randomised controlled trials. Br. J Cancer. 2020;123(11):1705-12.
Kuznia S, Zhu A, Akutsu T, Buring JE, Camargo CA, Jr., Cook NR, et al. Efficacy of vitamin d (3) supplementation on cancer mortality: Systematic review and individual patient data metaanalysis of randomised controlled trials. Ageing Res Rev. 2023;87:101923.
Wang L, Wang C, Wang J, Huang X, Cheng Y. Longitudinal, observational study on associations between postoperative nutritional vitamin d supplementation and clinical outcomes in esophageal cancer patients undergoing esophagectomy. Sci Rep. 2016;6:38962.
Madden JM, Murphy L, Zgaga L, Bennett K. De novo vitamin d supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res Treat. 2018;172(1):179-90.
Marshall DT, Savage SJ, Garrett-Mayer E, Keane TE, Hollis BW, Horst RL, et al. vitamin d 3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin. Endocrinol. Metab. 2012;97(7):2315-24.
Wagner D, Trudel D, Van der Kwast T, Nonn L, Giangreco AA, Li D, et al. Randomized clinical trial of vitamin d 3 doses on prostatic vitamin d metabolite levels and ki67 labeling in prostate cancer patients. J Clin. Endocrinol. Metab. 2013;98(4):1498-507.
Zeichner SB, Koru-Sengul T, Shah N, Liu Q, Markward NJ, Montero AJ, et al. Improved clinical outcomes associated with vitamin d supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin. Breast Cancer. 2015;15(1):e1-11.
Cadegiani FA. Remission of severe Myasthenia Gravis after massive-dose vitamin d treatment. Am. J. Case. Rep. 2016;17:51-4.
McCullough P, Amend J. Results of daily oral dosing with up to 60,000 international units (iu) of vitamin d 3 for 2 to 6 years in 3 adult males. J Steroid Biochem. Mol. Biol. 2017;173:308-12.
Amon U, Yaguboglu R, Ennis M, Holick MF, Amon J. Safety Data in Patients with Autoimmune Diseases during Treatment with High Doses of vitamin d 3 According to the "Coimbra Protocol". Nutrients. 2022;14(8).
Finamor DC, Sinigaglia-Coimbra R, Neves LC, Gutierrez M, Silva JJ, Torres LD, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin d on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5(1):222-34.
Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol. Endocrinol. 2012;48(3):R31-R43.
Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804-12.
Andrzejewski S, Siegel PM, St-Pierre J. Metabolic profiles associated with metforrmin efficacy in cancer. Front. Endocrinol. 2018;9:372.
Barrios-Bernal P, Zatarain-Barron ZL, Hernandez-Pedro N, Orozco-Morales M, Olivera-Ramirez A, Avila-Moreno F, et al. Will we unlock the benefit of metformin for patients with lung cancer? Lessons from current evidence and new hypotheses. Pharmaceuticals. 2022;15:786.
Saraei P, Asadi L, Kakar MA, Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Management and Research. 2019;11:3295-313.
Shi P, Liu W, Tala, Wang H, Li F, Zhang H, et al. Metformin suppresses triple-negative breast cancer stem cells by targeting KLF5 for degradation. Cell Discov. 2017;3:17010.
Lega IC, Shah PS, Margel D, Beyene J, Rochon PA, Lipscombe LL. The effect of metformin on mortality following cancer among patients with diabetes. Cancer Epidemiol. Biomarkers Prev. 2014;23(10):1974-84.
Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist. 2013;18(12):1248-55.
Mei ZB, Zhang ZJ, Liu CY, Liu Y, Cui A, Liang ZL, et al. Survival benefits of metformin for colorectal cancer patients with diabetes: a systematic review and meta-analysis. PloS ONE. 2014;9(3):e91818.
Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Annals of Oncolog. 2016;27:2184-95.
Eibl G, Rozengurt E. Metformin: Review of epidemiology and mechanisms of action in pancreatic cancer. Cancer Metastasis Rev. 2021;40:865-78.
Jimenez-Vacas JM, Herrero-Aguayo V, Montero-Hidalgo AJ, Saez-Martinez P, Gomez-Gomez E. Clinical, cellualr and molecular evidence of the additive antitumor effects of biguanides and statins in prostate cancer. Journal of Clinical Endocrinology & Metabolism. 2012;106:e696-e710.
Wang Y, Liu G, Tong D, Parmar H, Hasenmayer D, Yuan W, et al. Metformin represses androgen- dependent and androgen independnet prostrate cancers by targeting androgen receptor. Prostate. 2015;75:1187-96.
Buczynska A, Sidorkiewicz I, Kretowski AJ, Zbucka-Kretowska M, Adamska A. Metformin intervention - A panacea for cancer treatment? Cancers. 2022;14:1336.
Stopsack KH, Ziehr DR, Rider JR, Giovannucci EL. Metformin and prostate cancer mortality: a meta-analysis. Cancer Causes Control. 2016;27(1):105-13.
Giordano A, Tommonaro G. Curcumin and cancer. Nutrients. 2019;11:2376.
Pal S, Bhattacharyya S, Choudhuri T, Datta GK, Das T, Sa G. Amelioration of immune cell number depletion and potentiation of depressed detoxification system of tumor-bearing mice by curcumin. Cancer Detect. Prev. 2005;29(5):470-8.
Mansouri K, Rasoulpoor S, Daneshkhah A, Abolfathi S, Salari N, Mohammadi M, et al. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer. 2020;20(1):791.
Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old- age" disease with an "age-old" solution. Cancer Lett. 2008;267(1):133-64.
Santosa D, Suharti C, Riwanto I, Dharmana E, Pangarsa EA, Setiawan B, et al. Curcumin as adjuvant therapy to improve remission in myeloma patients: A pilot randomized clinical trial. Caspian. J Intern Med. 2022;13(2):375-84.
Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1b, IL-6, and TNF-a as well as cyclin E in TNF-a-treated HaCaT cells; NF-kB and MAPKs as potential upstream targets. Int. J Mol. Med. 2007;19(3):469-74.
Xiang DB, Zhang KQ, Zeng YL, Yan QZ, Shi Z, Tuo QH, et al. Curcumin: From a controversial "panacea" to effective antineoplastic products. Medicine (Baltimore). 2020;99(2):e18467.
Moghaddam SJ, Barta P, Mirabolfathinejad SG, Ammar-Aouchiche Z, Garza NT, Vo TT, et al. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30(11):1949-56.
Wang JY, Wang X, Wang XJ, Zheng BZ, Wang Y, Wang X, et al. Curcumin inhibits the growth via Wnt/B-catenin pathway in non-small-cell lung cancer cells. Eur Rev Med Pharmacol. Sci. 2018;22(21):7492-9.
Alexandrow MG, Song LJ, Altiok S, Gray J, Haura EB, Kumar NB. Curcumin: a novel Stat3 pathway inhibitor for chemoprevention of lung cancer. Eur J Cancer Prev. 2012;21(5):407-12.
Ye MX, Li Y, Yin H, Zhang J. Curcumin: updated molecular mechanisms and intervention targets in human lung cancer. Int. J Mol. Sci. 2012;13(3):3959-78.
Katta S, Srivastava A, Thangapazham RL, Rosner IL, Cullen J, Li H, et al. Curcumin-Gene Expression Response in Hormone Dependent and Independent Metastatic Prostate Cancer Cells. Int. J Mol. Sci. 2019;20(19).
Mach CM, Mathew L, Mosley SA, Kurzrock R, Smith JA. Determination of minimum effective dose and optimal dosing schedule for liposomal curcumin in a xenograft human pancreatic cancer model. Anticancer Res. 2009;29(6):1895-9.
Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010;173(5):590-601.
Panahi Y, Darvishi B, Ghanei M, Jowzi N, Beiraghdar F, Varnamkhasti BS. Molecular mechanisms of curcumins suppressing effects on tumorigenesis, angiogenesis and metastasis, focusing on NF-kB pathway. Cytokine Growth Factor Rev. 2016;28:21-9.
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14(14):4491-9.
Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase lia clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res (Phila). 2011;4(3):354- 64.
Li Y, Zhang T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014;346(2):197-205.
Zoi V, Galani V, Lianos GD, Voulgaris S, Kyritsis AP, Alexiou GA. The Role of Curcumin in Cancer Treatment. Biomedicines. 2021;9(9).
Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, et al. Targeting signal- transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann. N. Y. Acad. Sci. 2006;1091:151-69.
Pandey A, Vishnoi K, Mahata S, Tripathi SC, Misra SP, Misra V, et al. Berberine and Curcumin Target Survivin and STAT3 in Gastric Cancer Cells and Synergize Actions of Standard Chemotherapeutic 5-Fluorouracil. Nutr. Cancer. 2015;67(8):1293-304.
Yim-im W, Sawatdichaikul O, Semsri S, Horata N, Mokmak W, Tongsima S, et al. Computational analyses of curcuminoid analogs against kinase domain of HER2. BMC Bioinformatics. 2014;15(1):261.
Hu S, Xu Y, Meng L, Huang L, Sun H. Curcumin inhibits proliferation and promotes apoptosis of breast cancer cells. Exp Ther. Med. 2018;16(2):1266-72.
Wang K, Fan H, Chen Q, Ma G, Zhu M, Zhang X, et al. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs. 2015;26(1):15-24.
Starok M, Preira P, Vayssade M, Haupt K, Salome L, Rossi C. EGFR Inhibition by Curcumin in Cancer Cells: A Dual Mode of Action. Biomacromolecules. 2015;16(5):1634-42.
Sun XD, Liu XE, Huang DS. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol. Med Rep. 2012;6(6):1267-70.
Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, et al. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75(8):2077-82.
James MI, Iwuji C, Irving G, Karmokar A, Higgins JA, Griffin-Teal N, et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015;364(2):135-41.
Kunnumakkara AB, Harsha C, Banik K, Vikkurthi R, Sailo BL, Bordoloi D. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opinion on Drug Metabolism & Toxicology. 2019;15:705-33.
Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9(1):8-14.
Ghalaut VS, Sangwan L, Dahiya K, Ghalaut PS, Dhankhar R, Saharan R. Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J Oncol. Pharm Pract. 2012;18(2):186-90.
Hejazi J, Rastmanesh R, Taleban FA, Molana SH, Ehtejab G. A pilot clinical trial of radioprotective effects of curcumin supplementation in patients with prostate cancer. J. Cancer. Sci. Ther. 2013;5:320-4.
Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011;68(1):157-64.
Mahammedi H, Planchat E, Pouget M, Durando X, Cur© H, Guy L, et al. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology. 2016;90(2):69-78.
Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J Nutr. 2019;149(7):1133-9.
Pastorelli D, Fabricio ASC, Giovanis P, D'Ippolito S, Fiduccia P, Sold C, et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol. Res. 2018;132:72-9.
Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin. Oncol. 1997;15(6):2403-13.
Saghatelyan T, Tananyan A, Janoyan N, Tadevosyan A, Petrosyan H, Hovhannisyan A, et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine. 2020;70:153218.
Guorgui J, Wang R, Mattheolabakis G, Mackenzie GG. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin's lymphoma in mice. Arch Biochem. Biophys. 2018;648:12-9.
Moballegh Nasery M, Abadi B, Poormoghadam D, Zarrabi A, Keyhanvar P, Tavakol S, et al. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules. 2020;25:689.
Valizadeh H, Danshina S, Gencer MZ, Ammari A, Sadeghi A, Aslani S. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. International Immunopharmacology. 2020;89:107088.
Ahmadi R, Salari S, Reihani H, Eslami S. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebocontrolled clinical trial. Food Science & Nutrition. 2021;9:4068-75.
Rahimi HR, Nedaeinia R, Shamloo AS, Nikdoust S. Novel delivery system for natural products: Nano-curcumin formulations. AJP. 2016;6:383.
Skiba MB, Luis PB, Alfafara C, Billheimer D, Schneider C, Funk JL. Curcuminoid Content and Safety-Related Markers of Quality of Turmeric Dietary Supplements Sold in an Urban Retail Marketplace in the United States. Mol. Nutr. Food Res. 2018;62(14):e1800143.
Desai P, Ann D, Wang J, Prabhu S. Pancreatic Cancer: Recent Advances in Nanoformulation- Based Therapies. Crit Rev Ther. Drug Carrier Syst. 2019;36(1):59-91.
Nguyen HT, Phung CD, Thapa RK, Pham TT, Tran TH, Jeong JH, et al. Multifunctional nanoparticles as somatostatin receptor-targeting delivery system of polyaniline and methotrexate for combined chemo-photothermal therapy. Acta Biomater. 2018;68:154-67.
Tan BL, Norhaizan ME. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules. 2019;24(14).
Notice to US Food and Drug Administraion of the conclusion that the intended use of curcumin is generally recognized as safe. https://www.fda.gov/food/generally-recognized-safe-gras/gras- notice-inventory; 2018.
Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern. Med. 2006;6:10.
Panahi Y, Saadat A, Beiraghdar F, Nouzari SM, Jalalian HR. Antioxidant effects of bioavailability- enhanced curcuminoids in patients with solid tumors: A randomized double-blind placebocontrolled trial. Journal of Functional Foods. 2014;6:615-22.
Halegoua-Demarzio D, Navarro V, Ahmad J, Avula B, Barnhart H, Barritt AS, et al. Liver injury associated with tumeric - A growing problem: Ten cases from the drug-induced liver injury network [DILIN]. Am. J. Med. 2022.
Volak LP, Ghirmai S, Cashman JR, MH C. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab Dispos. 2008;36(8):1594-605.
Pavithra BH, Prakash N, Jayakumar K. Modification of pharmacokinetics of norfloxacin following oral administration of curcumin in rabbits. J Vet. Sci. 2009;10(4):293-7.
Kim DC, Ku SK, Bae JS. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012;45(4):221-6.
Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO) - mebendazole as an anti-cancer agent. ecancer. 2014;8:443.
Guerini AE, Triggiani L, Maddalo M, Bonu ML, Frassine F, Baiguini A, et al. Mebendazole as a candidate for drug repurposing in oncology: An extensive review of current literature. Cancers. 2019,'11:1284.
Meco D, Attina G, Mastrangelo S, Navarra P, Ruggiero A. Emerging perspectives on the antiparasitic Mebendazole as a repurposed drug for the treatment of brain cancers. Int. J. Mol. Sci. 2023;24:1334.
Nygren P, Larsson R. Drug repositioning from bench to bedside: tumour remission by the antihelmintic drug mebendazole in refractory metastatic colon cancer. Acta Oncol. 2014;53(3):427-8.
Dobrosotskaya IY, Hammer GD, Schteingart DE, Maturen KE, Worden FP. Mebendazole monotherapy and long-term disease control in metastatic adrenocortical carcinoma. Endocr. Pract. 2011;17(3):e59-e62.
Chiang RS, Syed AB, Wright JL, Montgomery B, Srinivas S. Fenbendazole enhancing anti-tumor effect: A case series. Clin. Oncol. Case Rep. 2021;4:2.
Sasaki JI, Ramesh R, Chada S, Gomyo Y, Roth JA, Mukhopadhyay T. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Molecular Cancer Therapeutics. 2002;2:1201-9.
Bai RY, Staedtke V, Rudin CM, Bunz F, Figgins GJ. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. NeuroOncology. 2015;17:545-54.
Doudican NA, Byron AA, Pollock PM, Orlow SJ. XIAP downregulation accompanies mebendazole growth inhibition in melanoma xenografts. Anti-Cancer Drugs. 2013;24:181-8.
Simbulan-Rosenthal CM, DDakshanamurthy S, Gaur A, Chen YS, Fang HB, Abdussamad M, et al. The repurposed anthelmintic mebendazole in combination with trametinib suppresses refractory NRASQ61k melanoma. Oncotarget. 2017;8:12576-95.
Walk-Vorderwulbecke V, Pearce K, Brooks T, Hubank M, Zwaan cM, Edwards AD, et al. Targeting acute myeloid leukemia by drug-induced c-MYB degradation. Leukemia. 2018;32:882-9.
Tan Z, Chen L, Zhang S. Comprehensive modeling and discovery of mebendazole as a novel TRAF2- and NCK-interacting kinase inhibitor. Scientific Reports. 2016;6:33534.
Pinto LC, Soares BM, de Jusus Viana Pinheiro J, Riggins GJ, Assumpcao PP, Burbano RM, et al.
The anthelmintic drug mebendazole inhibits growth, migration and invasion in gastric cancer cell model. Toxicology in Vitro. 2015;29:2038-44.
Pinto LC, de Fatima Aquino Moreira-Nunes C, Soares BM, Rodriguez Burbano RM, de Lemos JA, Montenegro R. Mebendazole, an antiparasitic drug, inhibits drug transporters expression in preclinical model of gastric peritoneal carcinomatosis. Toxicology in Vitro. 2017;43:87-91.
Nygren P, Fryknas M, Agerup B, Larsson R. Repositioning of the anthelmintic drug mebendazole for the treatment for colon cancer. J. Cancer res. Clin. Oncol. 2013;139:2133-40.
Gallia GL, Holdhoff M, Brem H, Joshi AD, Hann CL, Bai RY, et al. Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas: results of a phase 1 clinical trial. NeuroOncology Advances. 2021;3:1-8.
Xiong RG, Huang SY, Wu SX, Zhou DD, Yang ZJ, Saimaiti A, et al. Anticancer effects and mechanisms of berberine from medicinal herbs: An update review. Molecules. 2022; 27:4523.
Yao M, Fan X, Yuan B, Takagi N, Liu S, Han X, et al. Berberine inhibits NLRP3 inflammasone pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complementary and Alternative Medicine. 2019; 19:216.
Pan Y, Zhang F, Zhao Y, Shao D, Zheng X, Chen Y, et al. Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer. J. Cancer. 2017;8:1679-89.
Shu X, Li M, Cao Y, Li C, Zhou W, Ji G, et al. Berberine alleviates non-alcoholic steatohepatitis through modulating gut microbiota mediated intestinal FXR activation. Front. Pharmacol. 2021;12:750826.
Li S, Wang N, Tan HY, Chueng F, Zhang ZJ, Yuen MF, et al. Modulation of gut microbiota mediates berberine-induced expansion of immuno-suppressive cells to against alcoholic liver disease. Clinical and Translational Medicine. 2020;10:e112.
Zhu C, Li J, Hua Y, Wang J, Wang K, Sun J. Berberine inhibits the expression of SCT through miR- 214-3p stimulation in breast cancer cells. Evidence-Based Complementary and Alternative Medicine. 2020;2020:2817147.
Ruan H, Zhan YY, Hou J, Xu B, Chen B, Tian Y, et al. Berberine binds RXRalpha to suppress Beta- catenin signaling in colon cancer cells. Oncogene. 2017;36:6906-18.
Samad MA, Saiman MZ, Majid NA, Karsani SA, Yaacob JS. Berberine inhibits telomerase activity and induces cell cycle arrest and telomere erosion in colorectal cancer cell line, HCT 116. Molecules. 2021;26:376.
Zhao Z, Zeng J, Guo Q, Pu K, Yang Y, Chen N, et al. Berberine suppresses stemness and tumorigenicity of colorectal cancer stem-like cells by inhibiting m6 A methylation. Front. Oncol. 2021;11:775418.
Chen QQ, Shi JM, Ding Z, Xia Q, Zheng TS, Ren YB, et al. Berberine induces apoptosis in nonsmall-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Management and Research. 2019;11:9005-15.
Kou Y, Tong B, Wu W, Liao X, Zhao M. Berberine improves chemo-sensitivity to cisplatin by enhancing cell apoptosis and repressing PI3K/AKT/mTOR signaling pathway in gastric cancer. Front. Pharmacol. 2020;11:616251.
Dai W, Mu L, Cui Y, Li Y, Chen P, Xie H, et al. Berberine promotes apoptosis of colorectal cancer via regulation of the long non-coding RNA (IncRNA) cancer susceptibility candidate 2 (CASC2)/AU-biding factor 1 (AUF1)/Bcell CLL/Lymphoma 2 (Bcl-2) axis. Med. Sci. Monit. 2019;25:730-8.
Jeong Y, You D, Kang HG, Yu J, Kim SW, Nam SJ, et al. Berberine suppresses fibronectin expression through inhibition of c-jun phosphorylation in breast cancer cells. J. Breast Cancer. 2018;21:21-7.
Chu SC, Yu CC, Hsu LS, Chen KS, Su MY, Chen PN. Berberine reverses epithelial-to-mesenchymal transition and inhibits metastasis and tumor-induced angiogenesis in human cervical cancer cells. Mol. Pharmacol. 2014;86:609-23.
Liu CH, Tang WC, Sia P, Huang CC, Yang PM, Wu MH, et al. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesechchymal transition (EMT) associated genes with predictive and prognostic relevance. Int. J. Med. Sci. 2015;12:63-71.
Chen Y, Zhang H. Berberine and chemotherapeutic drugs synergistically inhibits cell proliferaion and migration of breast cancer cells. Int. J. Clin. Exp. Med. 2018;11:13243-50.
Zhao Y, Jing Z, Li Y, Mao W. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncology Reports. 2016;36:567-72.
Chen P, Dai CH, Shi ZH, Wang Y, Wu JN, Chen K, et al. Synergistic inhibitory effect of berberine and icotinib on non-small cell lung cancer cells via inducing autophagic cell death and apoptosis. Apoptosis. 2021;26:639-56.
You HY, Xie XM, Zhang WJ, Zhu HL, Jiang FZ. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cellular & Developmental Biology - Animal. 2016;52:857-63.
Chen YX, Gao QY, Zou TH, Wang BM, Liu SD, Sheng JQ, et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol. Hepatol. 2020;5(3):267-75.
Zhang Q, Wang X, Cao S, Sun Y, He X, Jiang B, et al. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomedicine and Pharmacotherapy. 2020;128:110245.
Parrales A, Thoenen E, Iwakuma T. The interplay between mutant p53 and the mevalonate pathway. Cell Death & Differentiation. 2017;25:460-70.
Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholestrol metabolism and cholestrol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 2013;4:119.
Borgquist S, Bjarnadottir O, Kimbung S, Ahern TP. Statins: a role in breast cancer therapy? J. Intern. Med. 2018;284:346-57.
Farwell WR, D'Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl. Cancer Inst. 2011;103(11):885-92.
Nelson JE, Harris RE. Inverse association of prostate cancer and non-steroidal anti-inflammatory drugs (NSAIDs): results of a case-control study. Oncol. Rep. 2000;7(1):169-70.
Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N. Engl. J Med. 2012;367(19):1792-802.
Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat. Rev. 2015;41(6):554-67.
Yu O, Eberg M, Benayoun S, Aprikian A, Batist G, Suissa S, et al. Use of statins and the risk of death in patients with prostate cancer. J Clin. Oncol. 2014;32(1):5-11.
Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int. J Cancer. 2016;139(6):1281-8.
Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl. Cancer Inst. 2011;103(19):1461-8.
Lash TL, Riis AH, Ostenfeld EB, Erichsen R, Vyberg M, Ahern TP, et al. Associations of Statin Use With Colorectal Cancer Recurrence and Mortality in a Danish Cohort. Am. J Epidemiol. 2017;186(6):679-87.
Shao JY, Lee FP, Chang CL, Wu SY. Statin-Based Palliative Therapy for Hepatocellular Carcinoma. Medicine (Baltimore). 2015;94(42):e1801.
Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Statin use and survival in colorectal cancer: Results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiol. 2016;45:71-81.
Lin JJ, Ezer N, Sigel K, Mhango G, Wisnivesky JP. The effect of statins on survival in patients with stage IV lung cancer. Lung Cancer. 2016;99:137-42.
Li L, Cui N, Hao T, Zou J, Wu J, Yi K, et al. Statins use and the prognosis of colorectal cancer: a meta-analysis. Clinics and Research in Hepatology and Gastroenterology. 2021;45:101588.
Ligibel JA, Bohlke K, May AM, Clinton SK, Demark-Wahnefried W, Gilchrist SC, et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J Clin. Oncol. 2022;40(22):2491-507.
Oberoi S, Robinson PD, Cataudella D, Culos-Reed SN, Davis H, Duong N, et al. Physical activity reduces fatigue in patients with cancer and hematopoietic stem cell transplant recipients: A systematic review and meta-analysis of randomized trials. Crit Rev Oncol. Hematol. 2018;122:52-9.
Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients With Cancer: A Systematic Review and MetaAnalysis. J Clin. Oncol. 2018;36(22):2297-305.
Garcia DO, Thomson CA. Physical activity and cancer survivorship. Nutr. Clin. Pract. 2014;29(6):768-79.
Aydin M, Kose E, Odabas I, Meric BB, Demirci D, Aydin Z. The Effect of Exercise on Life Quality and Depression Levels of Breast Cancer Patients. Asian Pac. J Cancer Prev. 2021;22(3):725-32.
Lopez P, Galvao DA, Taaffe DR, Newton RU, Souza G, Trajano GS, et al. Resistance training in breast cancer patients undergoing primary treatment: a systematic review and meta-regression of exercise dosage. Breast Cancer. 2021;28(1):16-24.
An KY, Morielli AR, Kang DW, Friedenreich CM, McKenzie DC, Gelmon K, et al. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: A randomized controlled trial. Int. J Cancer. 2020;146(1):150-60.
Bower JE, Partridge AH, Wolff AC, Thorner ED, Irwin MR, Joffe H, et al. Targeting Depressive Symptoms in Younger Breast Cancer Survivors: The Pathways to Wellness Randomized Controlled Trial of Mindfulness Meditation and Survivorship Education. J Clin Oncol. 2021;39(31):3473-84.
Gok Metin Z, Karadas C, Izgu N, Ozdemir L, Demirci U. Effects of progressive muscle relaxation and mindfulness meditation on fatigue, coping styles, and quality of life in early breast cancer patients: An assessor blinded, three-arm, randomized controlled trial. Eur J Oncol Nurs. 2019;42:116-25.
Greenlee H, DuPont-Reyes MJ, Balneaves LG, Carlson LE, Cohen MR, Deng G, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67(3):194-232.
Lundt A, Jentschke E. Long-Term Changes of Symptoms of Anxiety, Depression, and Fatigue in Cancer Patients 6 Months After the End of Yoga Therapy. Integr Cancer Ther. 2019;18:1534735418822096.
Büttner-Teleagä A, Kim YT, Osel T, Richter K. Sleep Disorders in Cancer-A Systematic Review. Int J Environ Res Public Health. 2021;18(21).
Chen Y, Tan F, Wei L, Li X, Lyu Z, Feng X, et al. Sleep duration and the risk of cancer: a systematic review and meta-analysis including dose-response relationship. BMC Cancer. 2018;18(1):1149.
Medysky ME, Temesi J, Culos-Reed SN, Millet GY. Exercise, sleep and cancer-related fatigue: Are they related? Neurophysiol Clin. 2017;47(2):111-22.
Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/B-catenin-mediated transcription in human breast tumor cells. Cancer Prev. Res (Phila). 2011;4(8):1275-84.
Chen L, Liu Y, Becher A, Diepold K, Schmid E, Fehn A, et al. Sildenafil triggers tumor lethality through altered expression of HSP90 and degradation of PKD2. Carcinogenesis. 2020;41(10):1421-31.
Chhonker SK, Rawat D, Koiri RK. Repurposing PDE5 inhibitor tadalafil and sildenafil as anticancer agent against hepatocellular carcinoma via targeting key events of glucose metabolism and multidrug resistance. J Biochem. Mol. Toxicol. 2022;36(8):e23100.
Islam BN, Sharman SK, Hou Y, Bridges AE, Singh N, Kim S, et al. Sildenafil Suppresses Inflammation-Driven Colorectal Cancer in Mice. Cancer Prev. Res (Phila). 2017;10(7):377-88.
Booth L, Roberts JL, Cruickshanks N, Conley A, Durrant DE, Das A, et al. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol. Pharmacol. 2014;85(3):408-19.
Booth L, Roberts JL, Cruickshanks N, Tavallai S, Webb T, Samuel P, et al. PDE5 inhibitors enhance celecoxib killing in multiple tumor types. J Cell Physiol. 2015;230(5):1115-27.
Domvri K, Zarogoulidis K, Zogas N, Zarogoulidis P, Petanidis S, Porpodis K, et al. Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer. J Cancer. 2017;8(18):3648-56.
Dent P, Booth L, Roberts JL, Poklepovic A, Hancock JF. (Curcumin+sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. J Cell Physiol. 2020;235(10):6862-74.
Tai LH, Alkayyal AA, Leslie AL, Sahi S, Bennett S, Tanese de SC, et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity.
Oncoimmunology. 2018;7(6):e1431082.
Cruz-Burgos M, Losada-Garcia A, Cruz-Hernandez CD, Cortes-Ramirez SA, Camacho-Arroyo I, Gonzalez-Covarrubias V, et al. New Approaches in Oncology for Repositioning Drugs: The Case of PDE5 Inhibitor Sildenafil. Front Oncol. 2021;11:627229.
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691-702.
Klutzny S, Anurin A, Nicke B, Regan JL, Lange M, Schulze L, et al. PDE5 inhibition eliminates cancer stem cells via induction of PKA signaling. Cell Death Dis. 2018;9(2):192.
Sutton SS, Magagnoli J, Cummings TH, Hardin JW. The Association Between Phosphodiesterase- 5 Inhibitors and Colorectal Cancer in a National Cohort of Patients. Clin. Transl. Gastroenterol. 2020;11(6):e00173.
Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid- derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 2015;21(1):39-48.
Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 2015;21(1):30-8.
Huang W, Sundquist J, Sundquist K, Ji J. Phosphodiesterase-5 inhibitors use and risk for mortality and metastases among male patients with colorectal cancer. Nat. Commun. 2020;11(1):3191.
Danley KT, Tan A, Catalona WJ, Leikin R, Helenowski I, Jovanovic B, et al. The association of phosphodiesterase-5 inhibitors with the biochemical recurrence-free and overall survival of patients with prostate cancer following radical prostatectomy. Urol. Oncol. 2022;40(2):57-.
Pantziarka P, Bouche G, Meheus L, Sukhatme S. Repurposing drugs in oncology (ReDO) - cimetidine as an anti-cancer agent. ecancer. 2014;8:485.
Aponte-Lopez A, Fuentes-PananÂj EM, Cortes-MuÂioz D, Munoz-Cruz S. Mast Cell, the Neglected Member of the Tumor Microenvironment: Role in Breast Cancer. J Immunol. Res. 2018;2018:2584243.
Ibrahim SSA, El-Aal SAA, Reda AM, Achy SE, Shahine Y. Anti-neoplastic action of Cimetidine/Vitamin C on histamine and the PI3K/AKT/mTOR pathway in Ehrlich breast cancer. Sci Rep. 2022;12(1):11514.
Liu FR, Jiang CG, Li YS, Li JB, Li F. Cimetidine inhibits the adhesion of gastric cancer cells expressing high levels of sialyl Lewis x in human vascular endothelial cells by blocking E-selectin expression. Int. J Mol. Med. 2011;27(4):537-44.
Kennedy L, Hodges K, Meng F, Alpini G, Francis H. Histamine and histamine receptor regulation of gastrointestinal cancers. Transl. Gastrointest. Cancer. 2012;1(3):215-27.
O'Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin. Immunol. 2011;128(6):1153-62.
Martin RK, Saleem SJ, Folgosa L, Zellner HB, Damle SR, Nguyen GK, et al. Mast cell histamine promotes the immunoregulatory activity of myeloid-derived suppressor cells. J Leukoc. Biol. 2014;96(1):151-9.
Katoh J, Tsuchiya K, Osawa H, Sato W, Matsumura G, Iida Y, et al. Cimetidine reduces impairment of cellular immunity after cardiac operations with cardiopulmonary bypass. J Thorac. Cardiovasc. Surg. 1998;116(2):312-8.
Cianchi F, Cortesini C, Schiavone N, Perna F, Magnelli L, Fanti E, et al. The role of cyclooxygenase-2 in mediating the effects of histamine on cell proliferation and vascular endothelial growth factor production in colorectal cancer. Clin. Cancer Res. 2005;11(19 Pt 1):6807-15.
Lin CY, Bai DJ, Yuan HY, Wang K, Yang GL, Hu MB, et al. Perioperative cimetidine administration promotes peripheral blood lymphocytes and tumor infiltrating lymphocytes in patients with gastrointestinal cancer: Results of a randomized controlled clinical trial. World J Gastroenterol. 2004;10(1):136-42.
TC vdPK, Snijders A, Boeije LC, De Groot ER, Alewijnse AE, Leurs R, et al. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J Clin. Invest. 1998;102(10):1866-73.
Caron G, Delneste Y, Roelandts E, Duez C, Bonnefoy JY, Pestel J, et al. Histamine polarizes human dendritic cells into Th2 cell-promoting effector dendritic cells. J Immunol. 2001;167(7):3682-6.
Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161(5):2586-93.
Ghosh AK, Hirasawa N, Ohuchi K. Enhancement by histamine of vascular endothelial growth factor production in granulation tissue via H(2) receptors. Br. J Pharmacol. 2001;134(7):1419-28.
Chihara Y, Fujimoto K, Miyake M, Hiasa Y, Hirao Y. Anti-tumor effect of cimetidine via inhibiting angiogenesis factors in N-butyl-N-(4-hydroxybutyl) nitrosamine-induced mouse and rat bladder carcinogenesis. Oncol. Rep. 2009;22(1):23-8.
Deva S, Jameson M. Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer. Cochrane Database Syst. Rev. 2012(8):CD007814.
Borgstrom S, von Eyben FE, Flodgren P, Axelsson B, Sjogren HO. Human leukocyte interferon and cimetidine for metastatic melanoma. N. Engl. J Med. 1982;307(17):1080-1.
Flodgren P, Borgstrom S, Jonsson PE, Lindstrom C, Sjogren HO. Metastatic malignant melanoma: regression induced by combined treatment with interferon [HuIFN-alpha(Le)] and cimetidine. Int. J Cancer. 1983;32(6):657-65.
Tonnesen H, Knigge U, Bulow S, Damm P, Fischerman K, Hesselfeldt P, et al. Effect of cimetidine on survival after gastric cancer. Lancet. 1988;2(8618):990-2.
Matsumoto S, Imaeda Y, Umemoto S, Kobayashi K, Suzuki H, Okamoto T. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. Br. J Cancer. 2002;86(2):161-7.
Adams WJ, Lawson JA, Morris DL. Cimetidine inhibits in vivo growth of human colon cancer and reverses histamine stimulated in vitro and in vivo growth. Gut. 1994;35(11):1632-6.
Kubota T, Fujiwara H, Ueda Y, Itoh T, Yamashita T, Yoshimura T, et al. Cimetidine modulates the antigen presenting capacity of dendritic cells from colorectal cancer patients. Br. J Cancer. 2002;86(8):1257-61.
Sarasola MP, TÄjquez Delgado MA, Nicoud MB, Medina VA. Histamine in cancer immunology and immunotherapy. Current status and new perspectives. Pharmacol. Res Perspect. 2021;9(5):e00778.
Breuer S, Maimon O, Appelbaum L, Peretz T, Hubert A. TL-118-anti-angiogenic treatment in pancreatic cancer: a case report. Med Oncol. 2013;30(2):585.
Niwa K, Onogi K, Wu Y, Mori H, Inoue Y, Tamaya T. Prognostic implications of cimetidine on advanced serous ovarian carcinoma related to cyclooxygenase-2 expression. Mol. Med Rep. 2008;1(1):119-22.
Fukuda M, Kusama K, Sakashita H. Cimetidine inhibits salivary gland tumor cell adhesion to neural cells and induces apoptosis by blocking NCAM expression. BMC Cancer. 2008;8:376.
Kinouchi T, Saiki S, Maeda O, Kuroda M, Usami M, Kotake T. Treatment of advanced renal cell carcinoma with a combination of human lymphoblastoid interfereon-alpha and cimetidine. J. Urol. 1997;157:1604-7.
Tatokoro M, Fujii Y, Kawakami S, Saito K, Koga F, Matsuoka Y, et al. Phase-II trial of combination treatment of interferon-alpha, cimetidine, cyclooxygenase-2 inhibitor and renin-angiotensin- system inhibitor (I-CCA therapy) for advanced renal cell carcinoma. Cancer Sci. 2011;102(1):137- 43.
Bobek V, Boubelik M, KovarÄ-k J, Taltynov O. Inhibition of adhesion breast cancer cells by anticoagulant drugs and cimetidine. Neoplasma. 2003;50(2):148-51.
Lefranc F, James S, Camby I, Gaussin JF, Darro F, Brotchi J, et al. Combined cimetidine and temozolomide, compared with temozolomide alone: significant increases in survival in nude mice bearing U373 human glioblastoma multiforme orthotopic xenografts. J Neurosurg. 2005;102(4):706-14.
Rok J, Rzepka Z, Kowalska J, Banach K, Beberok A, Wrzesniok D. The Anticancer Potential of Doxycycline and Minocycline-A Comparative Study on Amelanotic Melanoma Cell Lines. Int. J Mol. Sci. 2022;23(2).
Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br. J Pharmacol. 2013;169(2):337-52.
Rok J, Rzepka Z, Beberok A, Pawlik J, Wrzesniok D. Cellular and Molecular Aspects of AntiMelanoma Effect of Minocycline-A Study of Cytotoxicity and Apoptosis on Human Melanotic Melanoma Cells. Int. J Mol. Sci. 2020;21(18).
Rok J, Karkoszka M, Rzepka Z, Respondek M, Banach K, Beberok A, et al. Cytotoxic and proapoptotic effect of doxycycline - An in vitro study on the human skin melanoma cells. Toxicol. In Vitro. 2020;65:104790.
Weiler J, Dittmar T. Minocycline impairs TNF-a induced cell fusion of M13SV1-Cre cells with MDA-MB-435-pFDR1 cells by suppressing NF-kB transcriptional activity and its induction of target-gene expression of fusion-relevant factors. Cell Commun. Signal. 2019;17(1):71.
Lokeshwar BL. Chemically modified non-antimicrobial tetracyclines are multifunctional drugs against advanced cancers. Pharmacol. Res. 2011;63(2):146-50.
Niu G, Liao Z, Cai L, Wei R, Sun L. The combined effects of celecoxib and minocycline hydrochloride on inhibiting the osseous metastasis of breast cancer in nude mice. Cancer Biother. Radiopharm. 2008;23(4):469-76.
Gilbertson-Beadling S, Powers EA, Stamp-Cole M, Scott PS, Wallace TL, Copeland J, et al. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a nonmetalloproteinase-dependent mechanism. Cancer Chemother. Pharmacol. 1995;36(5):418-24.
Liu FY, Wu YH, Zhou SJ, Deng YL, Zhang ZY, Zhang EL, et al. Minocycline and cisplatin exert synergistic growth suppression on hepatocellular carcinoma by inducing S phase arrest and apoptosis. Oncol. Rep. 2014;32(2):835-44.
Masumori N, Tsukamoto T, Miyao N, Kumamoto Y, Saiki I, Yoneda J. Inhibitory effect of minocycline on in vitro invasion and experimental metastasis of mouse renal adenocarcinoma. J Urol. 1994;151(5):1400-4.
Markovic DS, Vinnakota K, van RN, Kiwit J, Synowitz M, Glass R, et al. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav. Immun. 2011;25(4):624-8.
Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, et al. The Role of Resveratrol in Cancer Therapy. Int. J Mol. Sci. 2017;18(12).
Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr. Pharm Des. 2013;19(34):6064-93.
Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269(2):243-61.
Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, et al. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J Cancer. 2010;127(2):257-68.
Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellon EA. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer- derived cell lines. J Androl. 2007;28(2):282-93.
Hsieh TC, Wong C, John BD, Wu JM. Regulation of p53 and cell proliferation by resveratrol and its derivatives in breast cancer cells: an in silico and biochemical approach targeting integrin avB3. Int. J Cancer. 2011;129(11):2732-43.
Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3'-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006;5(5):1335-41.
Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109(6):2293-302.
Zhang L, Wen X, Li M, Li S, Zhao H. Targeting cancer stem cells and signaling pathways by resveratrol and pterostilbene. Biofactors. 2018;44(1):61-8.
Vergara D, Valente CM, Tinelli A, Siciliano C, Lorusso V, Acierno R, et al. Resveratrol inhibits the epidermal growth factor-induced epithelial mesenchymal transition in MCF-7 cells. Cancer Lett. 2011;310(1):1-8.
Li C, Wang Q, Shen S, Wei X, Li G. HIF-1a/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells. Phytother. Res. 2019;33(3):798-807.
Fisher M, Knappertz V. The dose of aspirin for the prevention of cardiovascular and cerebrovascular events. Curr. Med Res Opin. 2006;22(7):1239-48.
Tao DL, Tassi YS, Williams CD, McCarty OJT. Aspirin and antiplatelet treatments in cancer. Blood. 2021;137(23):3201-11.
Negi RR, Rana SV, Gupta V, Gupta R, Chadha VD, Prasad KK, et al. Over-Expression of Cyclooxygenase-2 in Colorectal Cancer Patients. Asian Pac. J Cancer Prev. 2019;20(6):1675-81.
Wilson AJ, Fadare O, Beeghly-Fadiel A, Son DS, Liu Q, Zhao S, et al. Aberrant over-expression of COX-1 intersects multiple pro-tumorigenic pathways in high-grade serous ovarian cancer. Oncotarget. 2015;6(25):21353-68.
Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc. Natl. Acad. Sci U. S. A. 1998;95(2):681-6.
McCarty MF, Block KI. Preadministration of high-dose salicylates, suppressors of NF-kappa B activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy. Integr Cancer Ther. 2006;5(3):252-68.
Pan MR, Chang HC, Hung WC. Non-steroidal anti-inflammatory drugs suppress the ERK signaling pathway via block of Ras/c-Raf interaction and activation of MAP kinase phosphatases. Cell Signal. 2008;20(6):1134-41.
Thun MJ, Namboodiri MM, Heath CW, Jr. Aspirin use and reduced risk of fatal colon cancer. N. Engl. J Med. 1991;325(23):1593-6.
Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J Med. 2003;348(10):891-9.
Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J Med. 2003;348(10):883-90.
Gann PH, Manson JE, Glynn RJ, Buring JE, Hennekens CH. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl. Cancer Inst. 1993;85(15):1220-4.
Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47-55.
Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern Med. 2007;146(5):361-4.
Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741-50.
Chubak J, Whitlock EP, Williams SB, Kamineni A, Burda BU, Buist DS, et al. Aspirin for the Prevention of Cancer Incidence and Mortality: Systematic Evidence Reviews for the U.S. Preventive Services Task Force. Ann. Intern Med. 2016;164(12):814-25.
Chan AT, Ladabaum U. Where Do We Stand With Aspirin for the Prevention of Colorectal Cancer? The USPSTF Recommendations. Gastroenterology. 2016;150(1):14-8.
Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease
(ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036- 46.
McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of Aspirin on AllCause Mortality in the Healthy Elderly. N. Engl. J Med. 2018;379(16):1519-28.
McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N. Engl. J Med. 2018;379(16):1499-508.
Guirguis-Blake JM, Evans CV, Perdue LA, Bean SI, Senger CA. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2022;327(16):1585-97.
Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855-63.
Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J Med. 2020;382(11):1018-28.
Risch HA, Lu L, Streicher SA, Wang J, Zhang W, Ni Q, et al. Aspirin Use and Reduced Risk of Pancreatic Cancer. Cancer Epidemiol. Biomarkers Prev. 2017;26(1):68-74.
Wu D, Zhou B, Yang J, Qiu FB, Hu SY, Zhan HX. Can aspirin use reduce the risk of pancreatic cancer: an updated systematic review and meta-analysis. Journal of Pancreatology. 2020;3:201- 10.
Elwood PC, Morgan G, Delon C, Protty M, Galante J, Pickering J, et al. Aspirin and cancer survival: a systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers. Ecancermedicalscience. 2021;15:1258.
Wang X, Luo Y, Chen T, Zhang K. Low-dose aspirin use and cancer-specific mortality: a metaanalysis of cohort studies. J Public Health (Oxf). 2021;43(2):308-15.
Lebeau B, Chastang C, Muir JF, Vincent J, Massin F, Fabre C. No effect of an antiaggregant treatment with aspirin in small cell lung cancer treated with CCAVP16 chemotherapy. Results from a randomized clinical trial of 303 patients. The "Petites Cellules" Group. Cancer. 1993;71(5):1741-5.
Chen WY, Winder EP, Ballman KV, Winer EP, Openshaw TH, Hahn OM, et al. A randomized phase III, double-blinded, placebo-controlled trial of aspirin as adjuvent therapy for breast cancer (A011502): The aspirin after breast cancer (ABC) trial [abstract]. J. Clin. Oncol. 2022;40 (suppl):360922.
Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (reDO)- diclofenac as an anti-cancer agent. ecancer. 2023;10:610.
Giuliano F, Warner TD. Ex vivo assay to determine the cyclooxygenase selectivity of nonsteroidal anti-inflammatory drugs. Br. J Pharmacol. 1999;126(8):1824-30.
Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013;35(2):123-37.
Seed MP, Brown JR, Freemantle CN, Papworth JL, Colville-Nash PR, Willis D, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57(9):1625-9.
Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, et al. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003;197(2):221-32.
Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21-8.
Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71(24):7463-70.
Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int. Immunopharmacol. 2007;7(2):140-51.
Chesney JA, Mitchell RA, Yaddanapudi K. Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy. J Leukoc. Biol. 2017;102(3):727-40.
Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71(7):2664-74.
Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, BjÂ/nbeth BA, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol. Immunother. 2008;57(6):813-21.
Chirasani SR, Leukel P, Gottfried E, Hochrein J, Stadler K, Neumann B, et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int. J Cancer. 2013;132(4):843-53.
Inoue A, Muranaka S, Fujita H, Kanno T, Tamai H, Utsumi K. Molecular mechanism of diclofenac- induced apoptosis of promyelocytic leukemia: dependency on reactive oxygen species, Akt, Bid, cytochrome and caspase pathway. Free Radic. Biol Med. 2004;37(8):1290-9.
Gottfried E, Lang SA, Renner K, Bosserhoff A, Gronwald W, Rehli M, et al. New aspects of an old drug--diclofenac targets MYC and glucose metabolism in tumor cells. PloS ONE. 2013;8(7):e66987.
Sareddy GR, Kesanakurti D, Kirti PB, Babu PP. Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wntb-catenin/Tcf signaling pathway in human glioblastoma cells. Neurochem. Res. 2013;38(11):2313-22.
Gerthofer V, Kreutz M, Renner K, Jachnik B, Dettmer K, Oefner P, et al. Combined Modulation of Tumor Metabolism by Metformin and Diclofenac in Glioma. Int. J Mol. Sci. 2018;19(9).
Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth. Analg. 2010;110(6):1630-5.
Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De KM. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br. J Anaesth. 2014;113 Suppl 1:i82-i7.
Forget P, Bouche G, Duhoux FP, Coulie PG, Decloedt J, Dekleermaker A, et al. Intraoperative ketorolac in high-risk breast cancer patients. A prospective, randomized, placebo-controlled clinical trial. PloS ONE. 2019;14(12):e0225748.
Yi T, Cho SG, Yi Z, Pang X, Rodriguez M, Wang Y, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and ERK signaling pathways. Mol. Cancer Ther. 2008;7:1789-96.
Kundu J, Chun KS, Aruoma OI, Kundu JK. Mechanistic perpsectives on cancer chemoprevention/chemotherapeutic effects of thymoquinone. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2014;768:22-34.
Rahim MA, Shoukat A, Khalid W, Ejaz A, Itrat N, Majeed I, et al. A narrative review on various oil extraction methods, encapsulation processes, fatty acid profiles, oxidative stability, and medicinal properties of black seed (Nigella sativa). Foods. 2022;11:2826.
Mostofa AG, Hossain K, Basak D, Sayeed MS. Thymoquinone as a potential adjuvant therapy for cancer treatment: Evidence from preclinical studies. Front. Pharmacol. 2017;8:295.
Darakhshan S, Pou AB, Colagar AH, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacological Research. 2015;95:138-58.
Wei J, Wang B, Chen V, Wang Q, Ahmed AF, Zhang Y, et al. The immunomodulatory effects of active ingredients from Nigella sativa in RAW264.7 cells through NF-KB/MAPK signaling pathways. Front. Nutr. 2022;9:899797.
Majdalawich AF, Fayyad MW, Nasrallah GK. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Critical Reviews in Food Science and Nutrition. 2017;57:3911-28.
Zhao Z, Liu L, Li S, Hou X, Yang J. Advances in research on the relationship between thymoquinone and pancreatic cancer. Front. Oncol. 2023;12:1092020.
Majdalawieh AF, Fayyad MW. Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. Journal of Ayurevda and Integrative Medicine. 2016;7:173-80.
Johnson-Ajinwo OR, Ullah I, Mbye H, Richardson A, Horrocks P, Li WW. The synthesis and evaluation of thymoquinone analogues as anti-ovarian cancer and antimalarial agents. Biorganic & Medicinal Chemistry Letters. 2018;28:1219-22.
Ha JH, ayaraman M, adhakrishnan R, omathinayagam R, an M, ong YS. Differential effects of thymoquinone on lysophosphatidic acid-induced oncogenic pathways in ovarian cells. Journal of Traditional and Complementary Medicine. 2020;10:207-18.
El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int. J. Cancer. 2005;117:409-17.
Shariare MH, Khan A, Al-Masum A, Khan JH, Uddin J, Kazi M. Development of stable liposomal drug delivery system of thymoquinone and its In Vitro anticancer studies using breast cancer and cervical cancer cell lines. Molecules. 2022;27:6744.
Ng WK, Yazan LS, Ismail M. Thymoquinone from Nigella sativa was more potent than cisplatin in eliminating of SiHa cells via apoptosis with down-regulaiopn of Bcl-2 protein. Toxicology in Vitro. 2011;25:1392-8.
Alsanosi S, Sheikh RA, Sonbul S, Altayb HN, Batubara AS, Hosawani S, et al. The potential role of Nigella sativa seed oil as epigenetic therapy of cancer. Molecules. 2022;27:2779.
Elkady AI, Hussein RA, El-Assouli SM. Mechanism of action of Nigella sativa on human colon cancer cells: the suppression of AP-1 and NF-kB transcription factors and the inducion of cytoprotective genes. Asian Pac. J. Cancer. Prev. 2015;16:7943-57.
El-Far AH, Godugu K, Noreldin AE, Saddiq AA, Almaghrabi OA, Al Jaouni SK, et al. Thymoquinone and Costunolide induce apoptosis of both proliferative and doxorubin-induced-senescent colon and breast cancer cells. Integrative Cancer Therapies. 2021;30:1-20.
Abdualmjid RJ, Sergi CM. Mitochonfrial dysfunction and induction of apoptosis in hepatocellular carcinoma and cholangiocarcinoma cell lines by thymoquinone. Int. J. Mol. Sci. 2022;23:14669.
Thabrew MI, Mitry RR, Morsy MA, Hughes RD. Cytotoxic effects of a decoction of Nigella sativa, Hemidesmus indicus and Smalax glabra on human hepatoma HepG2 cells. Life Sci. 2005;77:1319-30.
Mbarek LA, Mouse HA, Elabbadi N, Bensalah M, Gamouh A, Aboufatima R, et al. Anti-tumor properties of blackseed (Nigella sativa L.) extracts. Brazilian Journal of Medical and Biological Research. 2007;40:839-47.
Khader M, Bresgen N, Eckl PM. Antimutagenic effects of ethanolic extracts from selected Palestinian medicinal plants. Journal of Ethnopharmacology. 2010;127:319-24.
Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, et al. Androgen receptor- and E2F-1-targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67:7782-8.
Shahraki S, Mohebbati R, Shafei MN, Mahmoudi M, Hosseinian S, Parhizgar S, et al. Induction of apoptosis and growth-inhibition by thymoquinone in ACHN and GP-293 cell lines in comparable with Cis-Platinum. Journal of Pharmacopuncture. 2019;22:176-83.
Chehi N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effectsof the Nigella Sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB. 2009;11:373-81.
Al-Sheddi ES, Farshori NN, Al-Oqail MM, Musarrat J, Al-Khedhairy AA, Siddiqui MA. Cytotoxicity of Nigella Sativa seed oil and extractt against human lung cancer cell line. Asian Pac. J. Cancer. Prev. 2023;15:983-7.
Kia ZA, Bizaki ST, Tapeh EA, Harijani SM, Katal N, Baziary RG. Recovering the angiogenic/angiostatic balance in NNK-induced lung carcinoma via 12 weeks of submaximal swimming and Nigella sativa nanocapsule. Toxicology Reports. 2022;9:1452-60.
Ayeka PA. Potential of Mushroom Compounds as Immunomodulators in Cancer Immunotherapy: A Review. Evid. Based Complement Alternat. Med. 2018;2018:7271509.
Park HJ. Current Uses of Mushrooms in Cancer Treatment and Their Anticancer Mechanisms.
Int. J Mol. Sci. 2022;23(18).
Dixon A, Elyaguov J, Choudhury M, Konno S. Anticancer effect of medicinal mushroom extract on renal cell carsinoma: Alternative therapeutic implication. World J. Nephrol. Urol. 2022;11:1-9.
Liu MM, Liu T, Yeung S, Wang Z, Andresen B, Parsa C, et al. Inhibitory activity of medicinal mushroom Ganoderma lucidum on colorectal cancer by attenuating inflammation. Precis. Clin. Med. 2021;4(4):231-45.
Cao Y, Xu X, Liu S, Huang L, Gu J. Ganoderma: A Cancer Immunotherapy Review. Front Pharmacol. 2018;9:1217.
Placido AI, roque F, Morgado M. The promising role of mushrooms as a therapeutic adjuvant of conventional cancer therapies. Biologics. 2022;2:58-68.
Jin X, Ruiz Beguerie J, Size D, Chan GC. Ganoderma lucidum (Reishi mushroom) for cancer treatment (Review). Cochrane Database of Syst. Rev. 2016;4:CD007731.
Zhong C, Li Y, Li W, Lian S, Li Y, Wu C, et al. Ganoderma lucidum extract promotes tumor cell pyroptosis and inhibits metastasis in breast cancer. Food Chem Toxicol. 2023;174:113654.
Kumagai Y, Akira S. Identification and functions of pattern-recognition receptors. J Allergy Clin. Immunol. 2010;125(5):985-92.
Wasser SP. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed J. 2014;37(6):345-56.
Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct. Target Ther. 2021;6(1):128.
Oka S, Tanaka S, Yoshida S, Hiyama T, Ueno Y, Ito M, et al. A water-soluble extract from culture medium of Ganoderma lucidum mycelia suppresses the development of colorectal adenomas. Hiroshima J Med Sci. 2010;59(1):1-6.
Chen X, Hu ZP, Yang XX, Huang M, Gao Y, Tang W, et al. Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int. Immunopharmacol. 2006;6(3):499-508.
Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol. Invest. 2003;32(3): 201-15.
Jeitler M, Michalsen A, Frings D, HA^bner M, Fischer M, Koppold-Liebscher DA, et al.
Significance of Medicinal Mushrooms in Integrative Oncology: A Narrative Review. Front Pharmacol. 2020;11:580656. Nowakowski P, Markiewicz-A»ukowska R, Bielecka J, Mielcarek K, Grabia M, Socha K. Treasures from the forest: Evaluation of mushroom extracts as anti-cancer agents. Biomed Pharmacother. 2021;143:112106.
Klupp NL, Chang D, Hawke F, Kiat H, Cao H, Grant SJ, et al. Ganoderma lucidum mushroom for the treatment of cardiovascular risk factors. Cochrane Database Syst. Rev. 2015;2015(2):CD007259.
Tang M, Hu X, Wang Y, Yao X, Zhang W. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacological Research. 2021;163:105207.
Juarez M, Schcolnik-Cabrera A, Duenas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018;8:317-31.
Dou Q, Chen HN, Wang K, Yuan K, Lei Y, Li K, et al. Ivermectin Induces Cytostatic Autophagy by Blocking the PAK1/Akt Axis in Breast Cancer. Cancer Res. 2016;76(15):4457-69.
Diao H, Cheng N, Zhao Y, Xu H, Dong H, Thamm DH, et al. Ivermectin inhibits canine mammary tumor growth by regulating cell cycle progression and WNT signaling. BMC Vet. Res. 2019;15(1):276.
Melotti A, Mas C, Kuciak M, Lorente-Trigos A, Borges I, Altaba A. The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol. Med. 2014;6(10):1263-78.
Diana A, Carlino F, Franzese E, Oikonomidou O, Criscitiello C, De VF, et al. Early Triple Negative Breast Cancer: Conventional Treatment and Emerging Therapeutic Landscapes. Cancers (Basel). 2020;12(4).
Kwon YJ, Petrie K, Leibovitch BA, Zeng L, Mezei M, Howell L, et al. Selective Inhibition of SIN3 Corepressor with Avermectins as a Novel Therapeutic Strategy in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2015;14(8):1824-36.
Chen L, Bi S, Wei Q, Zhao Z, Wang X. Ivermectin suppresses tumour growth and metastasis through degradation of PAK1 in esophageal squamous cell carcinoma. J. Cell. Mol. Med. 2020;24:5387-401.
Nappi L, Aguda AH, Nakouzi NA, Lelj-Garolla B, Beraldi E, Lallous N, et al. Ivermectin inhibits HSP27 and potentiates efficacy of oncogene targeting in tumor models. J Clin. Invest. 2020;130(2):699-714.
Sharmeen S, Skrtic M, Sukhai MA, Hurren R, Gronda M, Wang X, et al. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood. 2010;116(18):3593-603.
Draganov D, Han Z, Rana A, Bennett N, Irvine DJ, Lee PP. Ivermectin converts cold tumors hot and synergizes with immune checkpoint blockade for treatment of breast cancer. npj Beast Cancer. 2021;7:22.
de Castro CG, Gregianin LJ, Burger JA. Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection. Leuk. Lymphoma. 2020;61:2536-7.
Ishiguro T, Ishiguro RH, Ishiguro M, Toki A, Terunuma H. Synergistic Anti-tumor Effect of Dichloroacetate and Ivermectin. Cureus. 2022;14(2):e21884.
Spano D, Marshall JC, Marino N, De MD, Romano A, Scoppettuolo MN, et al. Dipyridamole prevents triple-negative breast-cancer progression. Clin. Exp Metastasis. 2013;30(1):47-68.
Gresele P, Momi S, Malvestiti M, Sebastiano M. Platelet-targeted pharmacologic treatments as anti-cancer therapy. Cancer Metastasis Rev. 2017;36(2):331-55.
Tsuruo T, Fujita N. Platelet aggregation in the formation of tumor metastasis. Proc. Jpn. Acad. Ser. B Phys. Biol Sci. 2008;84(6):189-98.
Gao J, Zhou C, Zhong Y, Shi L, Luo X, Su H, et al. Dipyridamole interacts with the N-terminal domain of HSP90 and antagonizes the function of the chaperone in multiple cancer cell lines. Biochem. Pharmacol. 2023;207:115376.
Budd GT, Herzog P, Bukowski RM. Phase I/II trial of dipyridamole, 5-fluorouracil, leukovorin, and mitoxantrone in metastatic breast cancer. Invest New Drugs. 1994;12(4):283-7.
Kohnoe S, Maehara Y, Takahashi I, Emi Y, Baba H, Sugimachi K. Treatment of advanced gastric cancer with 5-fluorouracil and cisplatin in combination with dipyridamole. Int. J Oncol. 1998;13(6):1203-6.
Raschko JW, Synold TW, Chow W, Coluzzi P, Hamasaki V, Leong LA, et al. A phase I study of carboplatin and etoposide administered in conjunction with dipyridamole, prochlorperazine and cyclosporine A. Cancer Chemother. Pharmacol. 2000;46(5):403-10.
Fleming RA, Capizzi RL, Muss HB, Smith S, Fernandes DJ, Homesley H, et al. Phase I study of N- (phosphonacetyl)-L-aspartate with fluorouracil and with or without dipyridamole in patients with advanced cancer. Clin. Cancer Res. 1996;2(7):1107-14.
Zasowska-Nowak A, Nowak PJ, Cialkowska-Rysz A. High-Dose Vitamin C in Advanced-Stage Cancer Patients. Nutrients. 2021;13(3).
Cameron E, Pauling L. Ascorbic acid and the glycosaminoglycans. An orthomolecular approach to cancer and other diseases. Oncology. 1973;27(2):181-92.
Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(10):3685-9.
Creagan ET, Moertel C, O'Fallon JR, Schuitt AJ, Rubin J, Frytak S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. New England Journal of Medicine. 1979;301(13):687-90.
Moertel CG, Fleming TR, Creagan ET, Rubin J, O'Connel MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. New England Journal of Medicine. 1985;312(3):137-41.
Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Annals of Internal Medicine. 2004;140(7):533-7.
Padayatty SJ, Levine M. Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds and serendipity. J Am. Coll. Nutr. 2000;19(4):423-5.
Leung PY, Miyashita K, Young M, Tsao CS. Cytotoxic effect of ascorbate and its derivatives on cultured malignant and nonmalignant cell lines. Anticancer Res. 1993;13(2):475-80.
Makino Y, Sakagami H, Takeda M. Induction of cell death by ascorbic acid derivatives in human renal carcinoma and glioblastoma cell lines. Anticancer Res. 1999;19(4B):3125-32.
Maramag C, Menon M, Balaji KC, Reddy PG, Laxmanan S. Effect of vitamin C on prostate cancer cells in vitro: effect on cell number, viability, and DNA synthesis. Prostate. 1997;32(3):188-95.
Davis JL, Paris HL, Beals JW, Binns SE, Giordano GR, Scalzo RL, et al. Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C bioavailability and capacity to protect against ischemia- reperfusion injury. Nutrition and Metabolic Insights. 2016;9:25-30.
Hickey S, Roberts HJ, Miller NJ. Pharmacokinetics of oral vitamin C. Journal of Nutritional & Environmental Medicine. 2008;17:169-77.
Mikirova N, Levy T, Hunningshake R. The levels of ascorbic acid in blood and mononuclear blood cells after oral liposome-encapsulated and oral non-encapsulated vitamin C supplementation, taken without and with IV hydrocortisone. J. Orthomol. Med. 2019;34.
Mikirova NA. Ascorbic Acid and Dehydroascorbic Acid Concentrations in Plasma and Peripheral Blood Mononuclear Cells after Oral Liposomal-Encapsulated or Intravenous Ascorbic Acid Delivery. J. Orthomol. Med. 2017;32:1-9.
Benade L, Howard T, Burk D. Synergistic killing of Ehrlich ascites carcinoma cells by ascorbate and 3-amino-1,2,4,-triazole. Oncology. 1969;23(1):33-43.
Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391-6.
Riordan HD, Riordan NH, Jackson JA, Casciari JJ, Hunninghake R, Gonzalez MJ, et al. Intravenous vitamin C as a chemotherapy agent: a report on clinical cases. P. R. Health Sci J. 2004;23(2):115- 8.
Gonzalez MJ, Berdiel MJ, Cintron AV. High dose IV vitamin C and metastatic breast cancer: A case report. J. Orthomol. Med. 2017;32:1.
Garcia KM, De Jesus C, Berdiel MJ, Miranda-Massari JR, Gonzalez MJ. Intravenous vitamin C and metabolic correction as adjuvant therapy for prostate cancer: a case report. J. Cancer Prev. Curr. Res. 2016;5:00164.
Nielsen TK, Hojgaard M, Andersen JT, Jorgensen NR, Zerahn B, Kristensen B, et al. Weekly ascorbic acid infusion in castration-resistant prostate cancer patients: a single-arm phase II trial. Transl. Androl Urol. 2017;6(3):517-28.
Wilson MK, Baguley BC, Wall C, Jameson MB, Findlay MP. Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac. J Clin. Oncol. 2014;10(1):22-37.
Carr AC, Cook J. Intravenous Vitamin C for Cancer Therapy - Identifying the Current Gaps in Our Knowledge. Front Physiol. 2018;9:1182.
Jacobs C, Hutton B, Ng T, Shorr R, Clemons M. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist. 2015;20(2):210-23.
Hoffer LJ, Robitaille L, Zakarian R, Meinychuk D, Kavan P, Agulnik J, et al. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: a phase I-II clinical trial. PloS ONE. 2015;10(4):e0120228.
Wang F, He MM, Xiao J, Zhang YQ, Yuan XL, Fang WJ, et al. A Randomized, Open-Label, Multicenter, Phase 3 Study of High-Dose Vitamin C Plus FOLFOX ± Bevacizumab versus FOLFOX ± Bevacizumab in Unresectable Untreated Metastatic Colorectal Cancer (VITALITY Study). Clin. Cancer Res. 2022;28(19):4232-9.
Stacpoole PW. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J Natl. Cancer Inst. 2017;109(11).
Abdel-Wahab AF, Mahmoud W, Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019;150:104511.
Albayrak G, Konac E, Dere UA, Emmez H. Targeting Cancer Cell Metabolism with Metformin, Dichloroacetate and Memantine in Glioblastoma (GBM). Turk. Neurosurg. 2021;31(2):233-7.
Powell SF, Mazurczak M, Dib EG, Bleeker JS, Geeraerts LH, Tinguely M, et al. Phase II study of dichloroacetate, an inhibitor of pyruvate dehydrogenase, in combination with chemoradiotherapy for unresected, locally advanced head and neck squamous cell carcinoma. Invest New Drugs. 2022;40(3):622-33.
Strum SB, Adalsteinsson O, Black RR, Segal D, Peress NL, Waldenfels J. Case report: Sodium dichloroacetate (DCA) inhibition of the "Warburg Effect" in a human cancer patient: complete response in non-Hodgkin's lymphoma after disease progression with rituximab-CHOP. J Bioenerg. Biomembr. 2013;45(3):307-15.
Khan A, Andrews D, Blackburn AC. Long-term stabilization of stage 4 colon cancer using sodium dichloroacetate therapy. World J Clin. Cases. 2016;4(10):336-43.
Khan A, Andrews D, Shainhouse J, Blackburn AC. Long-term stabilization of metastatic melanoma with sodium dichloroacetate. World J Clin. Oncol. 2017;8(4):371-7.
Brandsma D, Dorlo TP, Haanen JH, Beijnen JH, Boogerd W. Severe encephalopathy and polyneuropathy induced by dichloroacetate. J Neurol. 2010;257(12):2099-100.
Kinzel A, Ambrogi M, Varshaver M, Kirson ED. Tumor Treating Fields for Glioblastoma Treatment: Patient Satisfaction and Compliance With the Second-Generation Optune(®) System. Clin. Med Insights Oncol. 2019;13:1179554918825449.
Moser JC, Salvador E, Deniz K, Swanson K, Tuszynski J, Carlson KW, et al. The Mechanisms of Action of Tumor Treating Fields. Cancer Res. 2022;82(20):3650-8.
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318(23):2306-16.
Kim CY, Paek SH, Nam DH, Chang JH, Hong YK, Kim JH, et al. Tumor treating fields plus temozolomide for newly diagnosed glioblastoma: a sub-group analysis of Korean patients in the EF-14 phase 3 trial. J Neurooncol. 2020;146(3):399-406.
NCCN clinical practice guidelines in oncology, Central nervous systems cancer, Version 1. https://www.nccn.org/login?returnURL=https://www.nccn.org/professionals/physician gls/pdf/cns.pdf; 2018.
Ghiaseddin AP, Shin D, Melnick K, Tran DD. Tumor Treating Fields in the Management of Patients with Malignant Gliomas. Curr. Treat. Options Oncol. 2020;21(9):76.
Yanovsky RL, Bartenstein DW, Rogers GS, Isakoff SJ, Chen ST. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019;35(5):295- 303.
Dos Santos AF, de Almeida DR, Terra LF, Baptista M, Labriola L. Photodynamic therapy in cancer treatment - an update review. J. Cancer Metastasis Treat. 2019;5:25.
Reiter RJ, Ma Q, Sharma R. Melatonin in mitochondria: Mitigating clear and present dangers. Physiology. 2020;35:86-95.
Zimmerman S, Reiter RJ. Melatonin and the optics of the human body. Melatonin Res. 2019;2:138-60.
Hobday RA, Cason JW. The open-air treatment of pandemic influenza. Am. J. Public Health. 2022;99 Suppl.2:S236-S42.
Lindqvist PG, Epstein E, Landin-Olsson M, Ingvar C, Nielsen K, stenbeck M, et al. Avoidance of sun exposure is a risk factor for all-cause mortality: results form the Melanoma in Southern Sweden cohort. Journal of Internal Medicine. 2014;276:77-86.
Moore CM, Nathan TR, Lees WR, Mosse CA, Freeman A, Emberton M, et al. Photodynamic therapy using meso tetra hydroxy phenyl chlorin (mTHPC) in early prostate cancer. Lasers Surg. Med. 2006;38(5):356-63.
Dos Santos AF, Terra LF, Wailemann RA, Oliveira TC, Gomes VM, Mineiro MF, et al. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer. 2017;17(1):194.
Kostron H. Photodynamic diagnosis and therapy and the brain. In: Gomer CJ, ed. Photodynamic Therapy. Methods and Protocols: Humana Press; 2010:261-80.
Windahl T, Andersson SO, Lofgren L. Photodynamic therapy of localised prostatic cancer. Lancet. 1990;336(8723):1139.
Bredell MG, Besic E, Maake C, Walt H. The application and challenges of clinical PD-PDT in the head and neck region: a short review. J Photochem. Photobiol. B. 2010;101(3):185-90.
Moen I, Stuhr LE. Hyperbaric oxygen therapy and cancer--a review. Target Oncol. 2012;7(4):233- 42.
Raa A, Stansberg C, Steen VM, Bjerkvig R, Reed RK, Stuhr LE. Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA-induced rat mammary tumors. BMC Cancer. 2007;7:23.
Stuhr LE, Raa A, Oyan AM, Kalland KH, Sakariassen PO, Petersen K, et al. Hyperoxia retards growth and induces apoptosis, changes in vascular density and gene expression in transplanted gliomas in nude rats. J Neurooncol. 2007;85(2):191-202.
Gore A, Muralidhar M, Espey MG, Degenhardt K, Mantell LL. Hyperoxia sensing: from molecular mechanisms to significance in disease. J Immunotoxicol. 2010;7(4):239-54.
Moen I, Oyan AM, Kalland KH, Tronstad KJ, Akslen LA, Chekenya M, et al. Hyperoxic treatment induces mesenchymal-to-epithelial transition in a rat adenocarcinoma model. PloS ONE. 2009;4(7):e6381.
Poff AM, Ari C, Seyfried TN, D'Agostino DP. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PloS ONE. 2013;8(6):e65522.
Bennett MH, Feldmeier J, Smee R, Milross C. Hyperbaric oxygenation for tumour sensitisation to radiotherapy. Cochrane Database Syst. Rev. 2018;4(4):CD005007.
Webinar: Cancer Care and The Role of Repurposed Drugs - June 28, 2023
FLCC Weekly Especally the first 25 minutes
📄 Download the slides from VitaminDWiki
VitaminDWiki – Cancer category contains
{include}
VitaminDWiki – Cancer increases if poor Vitamin D Receptor
{include}
VitaminDWiki – Cancer - After diagnosis category contains
{include}
Vitamin D also PREVENTS some Cancers52+ studies
This list is automatically updated
{LIST()}
VitaminDWiki – WARNING: Chemotherapy and vitamin D - many studies
::Click on chart for details::
Many chemos are augmented by high-dose vitamin D
If the an augmented chemo dose is not reduced, the combination of Chemo + Vitamin D could be deadly
