Coimbra protocol using high-dose Vitamin D is safe
Safety Data in Patients with Autoimmune Diseases during Treatment with High Doses of Vitamin D3 According to the “Coimbra Protocol”
Nutrients 2022, 14(8), 1575; https://doi.org/10.3390/nu14081575
Ulrich Amon 1, Raul Yaguboglu 1. Madeleine Ennis 2. Michael F. Holick 3. And Julian Amon 1
1 International Centre for Skin Diseases DermAllegra, Coimbra Protocol Certified Center, Am Markgrafenpark 6, 91224 Pommelsbrunn-Hohenstadt, Germany
2 The Wellcome-Wolfson Institute for Experimental Medicine, Queens University of Belfast, Belfast BT7 1NN, UK
3 Endocrinology, Diabetes, Nutrition & Weight Management, Department of Medicine, Boston University School of Medicine, Boston, MA 02118, USA
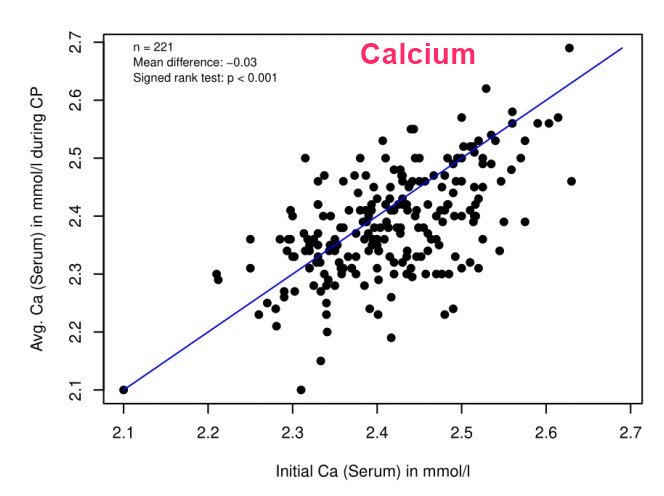
Calcium similar before and after, often drops a litte
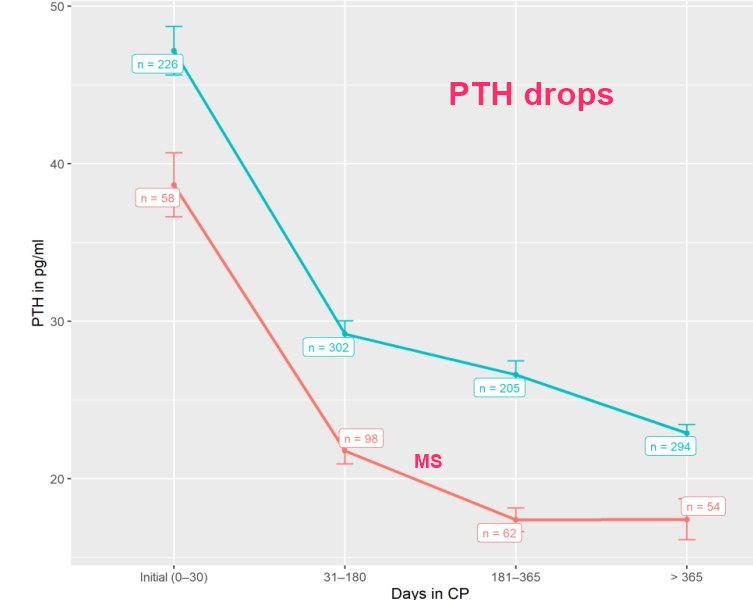
PTH drops a lot over time
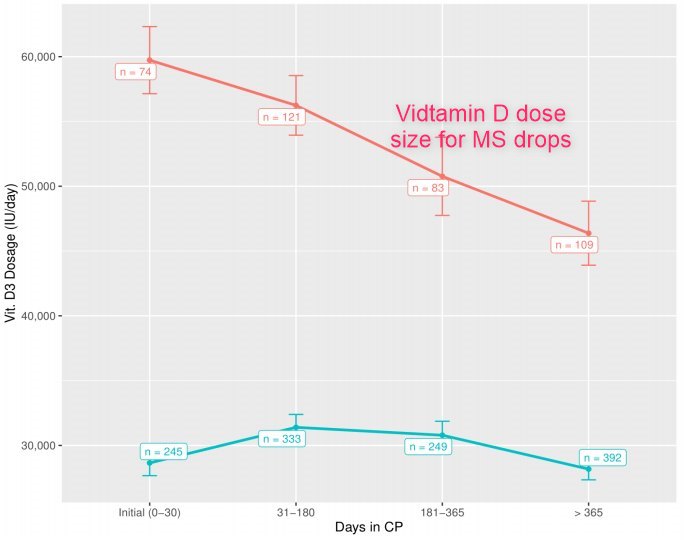
Dose size drops over time with MS
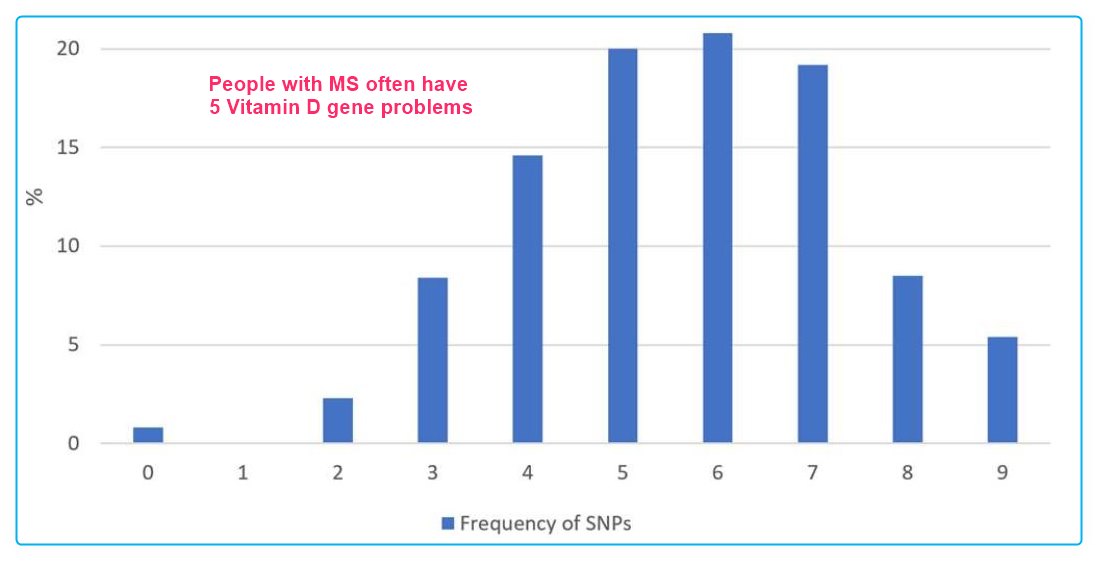
60% of MS patients have an average of 6 Vitamin D gene problems
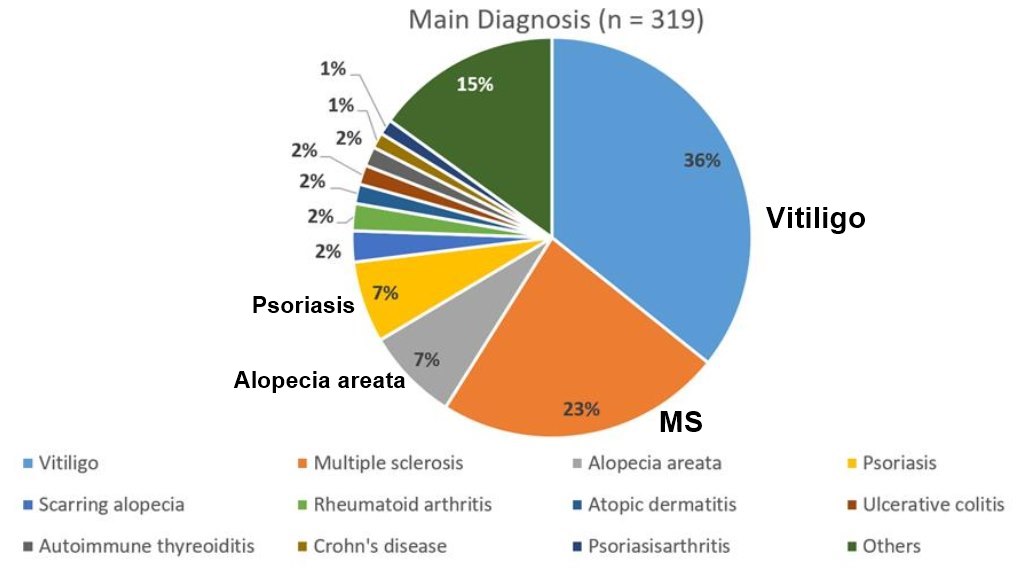
Principal Diagnoses: Vitiligo, MS, Psorasis, etc.
Background: In 2013, the group of Cicero Coimbra, Brazil, reported the clinical efficacy of high doses of vitamin D3 in patients suffering from autoimmune skin disorders (“Coimbra protocol”, CP). However, hypercalcemia and the subsequent impaired renal function may be major concerns raised against this protocol.
Methods: We report for the first time for a broad spectrum of autoimmune diseases in 319 patients (mean age (±SD) 43.3 ± 14.6 years, 65.5% female, 34.5% male) safety data for high doses of orally applied vitamin D3 (treatment period: up to 3.5 years) accompanied by a strict low-calcium diet and regular daily fluid intake of at least 2.5 L.
Results: Mean vitamin D3 dose was 35,291 ± 21,791 IU per day. The measurement of more than 6100 single relevant laboratory parameters showed all mean values (±SD) within the normal range for total serum calcium (2.4 ± 0.1 mmol/L), serum creatinine (0.8 ± 0.2 mg/dL), serum creatinine associated estimated GFR (92.5 ± 17.3 mL/min), serum cystatin C (0.88 ± 0.19 mg/L), serum TSH (1.8 ± 1 mIU/L), and for 24 h urinary calcium secretion (6.9 ± 3.3 mmol/24 h). We found a very weak relationship between the dosage of oral vitamin D3 and the subsequent calcium levels, both in serum and in urinary excretion over 24 h, respectively.
Conclusions: Our data show the reliable safety of the CP in autoimmune patients under appropriate supervision by experienced physicians.
📄 Download the PDF from VitaminDWiki
Discussion
Although the pathophysiological role and the therapeutic implications of vitamin D in autoimmunity are still under debate [6], the pleiotropic non-skeletal functions of vitamin D have been generally recognized for reducing the risk of complex non-communicable diseases (NCDs), including cardiovascular diseases, diabetes mellitus, depression, dementia, cancer, allergies, asthma as well as chronic infections [48-50].
While for most NCDs the putative pathophysiologic role of vitamin D as being an association or a cause-effect relationship is still unclear, recent evidence, using Mendelian randomization studies focusing on the genetic variants of vitamin D metabolism in order to reduce confounders, has confirmed an increased risk at least for MS in patients with vitamin D deficiency [51].
Therefore, MS might be the optimal "model" to study both the effects and safety parameters of the high-dose vitamin D treatment as offered with the CP [19]. To date, the treatment protocol of Cicero Coimbra in Brazil for a variety of autoimmune disorders, e.g., MS, psoriasis, and vitiligo, has only been published as pilot study [16], and randomized clinical trials are missing.
Patients with autoimmune diseases, especially MS, often look for holistic and alternative approaches in addition to symptomatic, immune suppressive and disease modifying treatments [52]. Nowadays, very extensive information is available in the World Wide Web [53-55]. There are many websites and personal case reports, which reflect the efficacy of CP as seen in social media by the patients [53-55].
Since the increasing interest in treatment with vitamin D and even ultra-high doses [7,56] results in the possible consequences of self-administering highly concentrated food supplements, we here primarily focus on the safety aspects of the CP.
In the pilot study of Coimbra's group published in 2013 [16], assessing the effect of the prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis, nine patients with psoriasis and sixteen patients with vitiligo received vitamin D3 35,000 IU once daily for six months in association with a consequent low-calcium diet (avoiding dairy products and calcium-enriched foods, such as oat, rice or soya "milk") and reliable hydration (minimum 2.5 L daily). In their study, serum creatinine and calcium (total and ionized) did not change and urinary calcium excretion increased within the normal range [16].
To our knowledge, we in this paper demonstrate for the first time for a broad spectrum of autoimmune diseases in over 300 patients with a treatment period of more than 3.5 years that high doses of orally applied vitamin D3 up to 1000 IU per kg bodyweight are safe in terms of calcium metabolism and renal function, when strict recommendations for diet and fluid intake are followed, up to a treatment period of 3.5 years.
With respect to our population, the mean daily dose of 35,000 IU Vitamin D3 is far higher than usual recommendations [57] and should be a contribution to the debate on the disparity of conclusions on what an "optimal" serum concentration of 25(OH)D is and how much supplementation is required to achieve a sufficient clinical response without long-term side effects.
For many years, we have been following clinically the recommendation of 25(OH)D ranging between 40 and 60 ng/mL being considered to be "optimal" (preferred range) for healthy people [9,58-60]. However, for autoimmune and inflammatory diseases, we have little knowledge about the variations of vitamin D metabolism and recognition, such as VDR polymorphisms, vitamin D binding protein polymorphisms, extrarenal 1 alpha hydroxylase activity, and micro RNAs [61].
Instead of solely focusing on 25(OH)D serum levels, different groups have recently elegantly worked out that, due to epigenetic and genetic differences, the individual immune responsiveness to vitamin D3 is rather complex [19,57,62,63]. Among other factors, this depends on the individual ability to convert vitamin D to its active metabolite 1,25(OH)2D and the interaction with VDR and the response elements [62-64]. Recently, it was shown that there is a dissociation between the calcemic and non-calcemic biologic actions of vitamin D3, especially on functions involved in immune activity [57].
Against this background, the suppression of PTH should be rather favored as a proxy for optimal vitamin D status as well as vitamin D3 treatment [65].
As expected [19], PTH levels dropped over time during CP treatment depending on the dose of vitamin D3 in our population. This confirms the preliminary results of Coimbra's group [16]: during a six month treatment period with a fixed daily dose of 35,000 IU vitamin D3 PTH levels significantly decreased from 57.8 ± 16.7 to 28.9 ± 8.2 pg/mL and from 55.3 ± 25.0 to 25.4 ± 10.7 pg/mL in patients with psoriasis and vitiligo, respectively.
Shirvani and co-workers [57] described a plateau in PTH levels in thirty randomized healthy adults at 16 weeks for a vitamin D3 dose of 4000 and 10,000 IU per day, but not for the group that received 600 IU per day, respectively, without changes in serum calcium. Interestingly, they observed a dose-dependent 25(OH)D alteration in broad gene expression with 162, 320 and 1289 genes up- or down-regulated in white blood cells, respectively [57].
However, due to higher oral vitamin D3 dosages in our patients suffering from MS, in our protocol, PTH plateaued at 15 pg/mL at approximately 6 months after start of treatment, which was different from non-MS autoimmune patients (Figure 5).
In comparison to healthy persons in autoimmunity genetic or epigenetic alterations of vitamin D metabolism differs both in quantity and quality of SNPs within genes of the vitamin D system (e.g., in activating enzymes, serum transport, and VDR) responsible for vitamin D status alterations, causing vitamin D resistance and reduced vitamin D responsiveness [7,19]. The influence of vitamin D3 high dose supplementation on genomewide expression in autoimmune patients remains unexplored.
As already stated in the pilot study of Finamor et al. [16], the daily requirements of vitamin D3 for patients with autoimmune disorders should be individually adapted to the profile of genetic polymorphisms of vitamin D metabolism.
The association between VDR, SNPs, and MS risk, for example, has been reported by many groups, whereas other vitamin D-related genes (including CYP2R1, CYP27B1, CYP24A1) have been less investigated [39].
In our population, the most frequent mutations were found in the genes encoding the enzymes 25-Hydroxylase (CYP2R1) and 1alpha-Hydroxylase (CYP27B1) with 75% of all patients, while SNPs in the regions of the VDR (BsmI, TaqI and FokI) were detected less frequently.
As previously shown, the genetic polymorphism of CYP27B1 associated with autoimmunity [66,67] causes a relative resistance to vitamin D requiring a higher level of circulating 25(OH)D3 to achieve biologically active 1,25(OH)2D3, resulting in normalized immune functions [16]. In order to achieve a physiologic rate of product formation in polymorphic enzyme variants, a higher Km (decreased affinity for substrate) and/or a lower Vmax require supra-physiologic concentrations of the substrate [16].
In patients with a combination of polymorphisms within different sections of vitamin D metabolism, this effect is potentiated. Supra-physiologic doses as applied according to CP may compensate for this genetic-related status of relative vitamin D resistance establishing tolerance to auto-antigens and may with respect to our safety data also increase tolerability in patients with autoimmune disorders [16]. This might explain the far lower PTH plateau in our patients in comparison with healthy subjects studied by Shirvani and co-workers [57].
According to Lemke et al. and the hypothesis of Cicero Coimbra of an acquired vitamin D resistance in autoimmune diseases [19], PTH concentrations could be used as a hallmark for individual adaption of oral vitamin D3 dosages. For an optimal physiological response of 1,25(OH)2D3, a low PTH plateau should reached and maintained within the lower third of the reference range [19]. With respect to our own experiences during the last four years, the degree of inflammation in autoimmune processes seem to influence the need to further decrease the PTH level by higher daily doses of vitamin D3.
A seven-year experience of McCullough et al. with oral vitamin D3 up to 50,000 IU per day did not reveal a linear or exponential relationship between vitamin D and calcium blood levels [68]. They did not observe cases of vitamin D3 induced hypercalcemia or any adverse events attributable to vitamin D3 supplementation in any patient.
Historically, many reports were published during the last century describing the successful use of vitamin D3, for example, in treating psoriasis, asthma, or rheumatoid arthritis with daily doses ranging from 60,000 to 300,000 IU [68]. Due to serious concerns following complications from vitamin-D-induced hypercalcemia after the prolonged daily intake of these supra-physiologic daily doses, vitamin D was then labelled as toxic [68].
With our current detailed knowledge about vitamin D metabolism, central cofactors (e.g., magnesium [69]), the influence of SNPs according to (epi)genetic studies [39], and worldwide experience of several thousands of patients treated with the CP from 2012 onwards with daily doses up to 340,000 IU [70], we are able to develop an individualized vitamin D3 treatment for autoimmune patients by the careful planning and determination of reliable mechanisms for regular laboratory controls. Based on our findings, hypercalcemia does not appear to be a first line risk of high-dose vitamin D3 therapy.
However, since hypervitaminosis D leads to increased calcium absorption via the upregulation of intestinal VDR, a strict calcium-reduced diet is mandatory to protect patients from hypercalcemia. At baseline, patients must be informed in great detail about this central aspect. Restrictions in milk, dairy products and calcium-enriched food stuff have contributed to minimize the calciotropic effects of high daily doses of vitamin D3 in the current study, which confirms the data of the pilot study of Coimbra's group [16]. We strongly recommend that CP is always used in the hands of qualified and experienced physicians and strongly advise against the use of CP by patients themselves based on Internet information.
Among all the chronic inflammatory skin diseases we previously studied (Amon U et al., 2018), the average 25(OH)D serum level was lowest in patients with plaque psoriasis (psoriasis vulgaris), only in patients with severe acute or chronic recurrent skin infections on the skin did we find significantly lower levels. Since the 1930s [71], successful oral substitution of vitamin D in psoriasis patients has been demonstrated in different studies [72]. Since for psoriasis, an increased incidence of other inflammatory comorbidities (e.g., psoriatic arthritis, arteriosclerosis, diabetes mellitus, obesity, non-alcoholic steatohepatitis, inflammatory bowel diseases, and depression) has been described [73], for which—independent of psoriasis—a modulating role of vitamin D has also been discussed in recent publications [74], we strongly recommend that physicians consider using the CP for patients suffering from psoriasis [16].
Perez and co-workers observed an improvement of 88% after oral 1,25(OH)2D3 treatment in 85 patients with psoriasis, with approximately 27% of patients healing completely, 36% having a moderate and 25% showing a slight improvement without causing hypercalcemia [75]. High oral doses of vitamin D3 (35,000 IU once daily) for six months led to a significant improvement of Psoriasis Area and Severity Index (PASI) score in nine patients with psoriasis without significant side effects [75]. Earlier work has also demonstrated a drastic improvement following the oral administration of vitamin D2 in severe cases [71] and following the use of 1a-hydroxyvitamin D3 and 1,25(OH)D3 [76,77].
In our analysis, vitiligo was the dominant diagnosis. Vitiligo is a polygenic autoimmune disease, characterized by localized or generalized depigmentation of the skin as a result of melanocyte destruction by immunologic dysfunction [78]. Two recent metaanalyses identified a significant positive relationship between lower 25(OH)D serum levels and the incidence of vitiligo [79,80]. In our recent study, among 113 patients with vitiligo, 41.2% had a 25(OH)D serum level below 20 ng/mL at baseline before treatment [11].
Zhang et al. performed a meta-analysis with 17 studies on VDR gene polymorphisms (BsmI, ApaI, TaqI, and FokI) in vitiligo, resulting in an increased susceptibility risk of vitiligo only for the Apal polymorphism of the VDR; BsmI, TaqI, and FokI loci had no obvious correlation [80]. In our study, FokI (rs2228570) was most often mutated in comparison to BsmI and TaqI.
When Finamor et al. treated 16 vitiligo patients with daily doses of 35,000 IE vitamin D3 over a period of six months, no repigmentation of the affected areas was observed in only five patients, but the others did develop repigmentation up to 75% without further dermatological treatment [16]. In our center, the combination of CP with topical treatment (calcineurin inhibitors) and/or 311 nm narrow band UVB or 308 nm excimer laser regularly leads to repigmentation in all patients, of course to a varying extent, which will be reported elsewhere. With respect to the data presented here, the vast majority of our vitiligo patients had a daily dose of vitamin D3 not higher than 40,000 IE. For non-inflammatory autoimmune skin diseases (such as vitiligo or alopecia areata), in general, a lower vitamin D3 dose appears to be sufficient for a good clinical response (drop of PTH levels in patients with vitiligo: baseline vs. >365 days p < 0.001, non-vitiligo patients: baseline vs. >365 days p < 0.001), as has been suggested elsewhere [16,19].
Despite our evidence that high-dose vitamin D3 application according to CP is generally well tolerated without signs for long term toxicity, this therapeutic approach of vitamin D3 supplementation should be embedded in a holistic treatment package. Against this background, fascinating new findings from gut microbiome research demonstrated variations in the vitamin D receptor also influence the large functional network of gut microbiota [81].
Finally, further possible significant factors influencing vitamin D sensitivity and metabolism as well as disease activity of autoimmune processes, such as gender differences [82] and the degree of chronic psychoneuroimmunologic impact [83], should be elaborated in further studies, when the clinical efficacy of CP is evaluated in detail. It will be also particularly important to differentiate the clinical effects between the different autoimmune diseases treated by the regimen.
Conclusions
In summary, to our knowledge, our work provides the first long-term documentation of selected critical laboratory parameters during the application of the CP using a high-dose oral vitamin D3 in a broad spectrum of different autoimmune diseases, demonstrating that this procedure is well tolerated with respect to renal function and calcium metabolism. In terms of individualized treatment, we suggest to further use serum levels of PTH as biomarker for an individual response to vitamin D3, the individual ability to convert vitamin D to the active metabolite, the 1,25(OH)2D's interaction with its receptor and the response elements and finally the differential supplementation with vitamin D3. In further studies, possible differences of the clinical outcome of CP treatment should be investigated.
References
1. Cooper, G.S.; Stroehla, B.C. The Epidemiology of Autoimmune Diseases. Autoimmun. Rev. 2003,2,119-125. [CrossRef]
2. Rose, N.R. Prediction and Prevention of Autoimmune Disease in the 21st Century: A Review and Preview. Am. J. Epidemiol. 2016, 183, 403-406. [CrossRef] [PubMed]
3. Rosenblum, M.D.; Gratz, I.K.; Paw, J.S.; Abbas, A.K. Treating Human Autoimmunity: Current Practice and Future Prospects. Sci. Transl. Med. 2012, 4,125sr1. [CrossRef] [PubMed]
4. Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of "Western Diet" in Inflammatory Autoimmune Diseases. Curr. Allergy Asthma Rep. 2013,14, 404. [CrossRef] [PubMed]
5. Vojdani, A.; Pollard, K.M.; Campbell, A.W. Environmental Triggers and Autoimmunity. Autoimmune Dis. 2014, 2014, 798029. [CrossRef] [PubMed]
6. Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020,12, 789. [CrossRef] [PubMed]
7. Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020,12, 2097. [CrossRef]
8. Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T Cells. Nutrients 2015, 7, 3011-3021. [CrossRef]
9. Wacker, M.; Holick, M. Vitamin D-Effects on Skeletal and Extraskeletal Health and the Need for Supplementation. Nutrients 2013, 5,111-148. [CrossRef]
10. Hollis, B.W.; Wagner, C.L. The Role of the Parent Compound Vitamin D with Respect to Metabolism and Function: Why Clinical Dose Intervals Can Affect Clinical Outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619-4628. [CrossRef]
11. Amon, U.; Baier, L.; Yaguboglu, R.; Ennis, M.; Holick, M.F.; Amon, J. Serum 25-Hydroxyvitamin D Levels in Patients with Skin Diseases Including Psoriasis, Infections, and Atopic Dermatitis. Dermato-Endocrinology 2018,10, e1442159. [CrossRef] [PubMed]
12. Norman, A.W.; Bouillon, R. Vitamin D Nutritional Policy Needs a Vision for the Future. Exp. Biol. Med. 2010, 235,1034-1045. [CrossRef] [PubMed]
13. Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 Positivity Rates Associated with Circulating 25-Hydroxyvitamin D Levels. PLoS ONE 2020,15, e0239252. [CrossRef] [PubMed]
14. Mark, K.A.; Dumas, K.J.; Bhaumik, D.; Schilling, B.; Davis, S.; Oron, T.; Sorensen, D.J.; Lucanic, M.; Brem, R.B.; Melov, S.; et al. Vitamin D Promotes Protein Homeostasis and Longevity via the Stress Response Pathway Genes Skn-1, Ire-1, and Xbp-1. Cell Rep. 2016, 17, 1227-1237. [CrossRef]
15. Caristia, S.; Filigheddu, N.; Barone-Adesi, F.; Sarro, A.; Testa, T.; Magnani, C.; Aimaretti, G.; Faggiano, F.; Marzullo, P. Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies. Nutrients 2019,11, 2384. [CrossRef]
16. Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.M.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A Pilot Study Assessing the Effect of Prolonged Administration of High Daily Doses of Vitamin D on the Clinical Course of Vitiligo and Psoriasis. Dermato-Endocrinology 2013, 5, 222-234. [CrossRef]
17. Available online: https://www.coimbraprotocol.com/the-protocol-1 (accessed on 19 February 2022).
18. Available online: https://coimbraprotokoll.de/coimbra/ (accessed on 19 February 2022).
19. Lemke, D.; Klement, R.J.; Schweiger, F.; Schweiger, B.; Spitz, J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front. Immunol. 2021,12, 655739. [CrossRef]
20. Available online: https://coimbraprotokoll.de/coimbraprotokoll_aerzte/ (accessed on 19 February 2022).
21. Charlon, T.; Martinez-Bueno, M.; Bossini-Castillo, L.; Carmona, F.D.; Di Cara, A.; Wojcik, J.; Voloshynovskiy, S.; Martín, J.; Alarcon-Riquelme, M.E. Single Nucleotide Polymorphism Clustering in Systemic Autoimmune Diseases. PLoS ONE 2016, 11, e0160270. [CrossRef]
22. Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of Vitamin D Metabolism Gene Polymorphisms with Autoimmunity: Evidence in Population Genetic Studies. Int. J. Mol. Sci. 2020, 21, 9626. [CrossRef]
23. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 19 February 2022).
24. Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018,118, 181-189. [CrossRef]
25. Whang, R. Magnesium Deficiency: Pathogenesis, Prevalence, and Clinical Implications. Am. J. Med. 1987, 82, 24-29. [CrossRef]
26. Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin Effects on the Immune System: Vitamins a and D Take Centre Stage. Nat. Rev. Immunol. 2008, 8, 685-698. [CrossRef] [PubMed]
27. Oliveira, L.D.M.; Teixeira, F.M.E.; Sato, M.N. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 3067126. [CrossRef] [PubMed]
28. Hufnagl, K.; Jensen-Jarolim, E. Vitamin a and D in Allergy: From Experimental Animal Models and Cellular Studies to Human Disease. Allergo J. Int. 2018, 27, 72-78. [CrossRef]
29. Gröber, U. Vitamin a (Retinol). Z. Für Orthomol. Med. 2019,17, 44-49. [CrossRef]
30. Reza Dorosty-Motlagh, A.; Mohammadzadeh Honarvar, N.; Sedighiyan, M.; Abdolahi, M. The Molecular Mechanisms of Vitamin a Deficiency in Multiple Sclerosis. J. Mol. Neurosci. 2016, 60, 82-90. [CrossRef]
31. Fragoso, Y.D.; Stoney, P.N.; McCaffery, P.J. The Evidence for a Beneficial Role of Vitamin a in Multiple Sclerosis. CNS Drugs 2014, 28, 291-299. [CrossRef]
32. Gerster, H. Vitamin A-functions, dietary requirements and safety in humans. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J. Int. De Vitaminol. Et De Nutr. 1997, 67, 71-90.
33. Rheaume-Bleue, K. Vitamin K2 and the Calcium Paradox: How a Little-Known Vitamin Could Save Your Life; John Wiley & Sons: Hoboken, NJ, USA, 2011.
34. Beulens, J.W.J.; Booth, S.L.; van den Heuvel, E.G.H.M.; Stoecklin, E.; Baka, A.; Vermeer, C. The Role of Menaquinones (Vitamin K2) in Human Health. Br. J. Nutr. 2013,110,1357-1368. [CrossRef]
35. Epstein, M. Matrix Gla-Protein (MGP) Not Only Inhibits Calcification in Large Arteries but Also May Be Renoprotective: Connecting the Dots. EBioMedicine 2016, 4,16-17. [CrossRef]
36. Li, J.; Wang, H.; Rosenberg, P.A. Vitamin K Prevents Oxidative Cell Death by Inhibiting Activation of 12-Lipoxygenase in Developing Oligodendrocytes. J. Neurosci. Res. 2009, 87,1997-2005. [CrossRef]
37. Lasemi, R.; Kundi, M.; Moghadam, N.B.; Moshammer, H.; Hainfellner, J.A. Vitamin K2 in Multiple Sclerosis Patients. Wien. Klin. Wochenschr. 2018,130, 307-313. [CrossRef] [PubMed]
38. Price, P.A.; Faus, S.A.; Williamson, M.K. Warfarin-Induced Artery Calcification Is Accelerated by Growth and Vitamin D. Arterioscler. Thromb. Vasc. Biol. 2000, 20,317-327. [CrossRef] [PubMed]
39. Scazzone, C.; Agnello, L.; Bivona, G.; Lo Sasso, B.; Ciaccio, M. Vitamin D and Genetic Susceptibility to Multiple Sclerosis. Biochem. Genet. 2020, 59,1-30. [CrossRef]
40. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2060793 (accessed on 19 February 2022).
41. Available online: https://www.ncbi.nlm.nih.gov/snp/rs703842 (accessed on 19 February 2022).
42. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2296241 (accessed on 19 February 2022).
43. Available online: https://www.ncbi.nlm.nih.gov/snp/rs7041 (accessed on 19 February 2022).
44. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1155563 (accessed on 19 February 2022).
45. Available online: https://www.ncbi.nlm.nih.gov/snp/rs4588 (accessed on 19 February 2022).
46. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1544410 (accessed on 19 February 2022).
47. Available online: https://www.ncbi.nlm.nih.gov/snp/rs731236 (accessed on 19 February 2022).
48. Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D Status and Ill Health: A Systematic Review. Lancet Diabetes Endocrinol. 2014, 2, 76-89. [CrossRef]
49. Holick, M.F. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer. Res. 2016, 36, 345-1356.
50. Holick, M.F. Can You Have Your Cake and Eat It Too? The Sunlight D-Lema. Br. J. Dermatol. 2016,175,1129-1131. [CrossRef]
51. Mokry, L.E.; Ross, S.; Ahmad, O.S.; Forgetta, V.; Smith, G.D.; Leong, A.; Greenwood, C.M.T.; Thanassoulis, G.; Richards, J.B. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. PLOS Med. 2015,12, e1001866. [CrossRef]
52. Schwartz, C.E.; Vollmer, T.; Lee, H. Reliability and Validity of Two Self-Report Measures of Impairment and Disability for MS. Neurology 1999, 52, 63. [CrossRef]
53. Available online: https://www.nationalmssociety.org/Treating-MS/Complementary-Alternative-Medicines (accessed on 20 February 2022).
54. Available online: https://www.facebook.com/coimbraprotocol.vitamind (accessed on 20 February 2022).
55. Available online: https://www.coimbraprotocol.com/testimonials-1 (accessed on 20 February 2022).
56. Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020,12, 783. [CrossRef]
57. Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D's Calcemic Activity and Non-Calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019,9. [CrossRef] [PubMed]
58. Veugelers, P.; Pham, T.-M.; Ekwaru, J. Optimal Vitamin D Supplementation Doses That Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population. Nutrients 2015, 7,10189-10208. [CrossRef] [PubMed]
59. Lugg, S.T.; Howells, P.A.; Thickett, D.R. Optimal Vitamin D Supplementation Levels for Cardiovascular Disease Protection. Disease Markers 2015, 2015,864370. [CrossRef] [PubMed]
60. Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96,1911-1930. [CrossRef]
61. Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common Genetic Determinants of Vitamin D Insufficiency: A Genome-Wide Association Study. Lancet 2010, 376, 180-188. [CrossRef]
62. Carlberg, C.; Haq, A. The Concept of the Personal Vitamin D Response Index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12-17. [CrossRef]
63. Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019,11, 676. [CrossRef]
64. Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of Vitamin D Status and Vitamin D3 Supplementation on Genome Wide Expression of White Blood Cells: A Randomized Double-Blind Clinical Trial. PLoS ONE 2013, 8, e58725. [CrossRef]
65. Mendes, M.M.; Hart, K.H.; Lanham-New, S.A.; Botelho, P.B. Suppression of Parathyroid Hormone as a Proxy for Optimal Vitamin D Status: Further Analysis of Two Parallel Studies in Opposite Latitudes. Nutrients 2020,12, 942. [CrossRef]
66. Pani, M.; Regulla, K.; Segni, M.; Krause, M.; Hofmann, S.; Hufner, M.; Herwig, J.; Pasquino, A.; Usadel, K.; Badenhoop, K. Vitamin D lalpha-Hydroxylase (CYPlalpha) Polymorphism in Graves' Disease, Hashimoto's Thyroiditis and Type 1 Diabetes Mellitus. Eur. J. Endocrinol. 2002,146, 777-781. [CrossRef]
67. Sundqvist, E.; Bäärnhielm, M.; Alfredsson, L.; Hillert, J.; Olsson, T.; Kockum, I. Confirmation of Association between Multiple Sclerosis and CYP27B1. Eur. J. Hum. Genet. 2010,18,1349-1352. [CrossRef] [PubMed]
68. McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily Oral Dosing of Vitamin D3 Using 5000 to 50,000 International Units a Day in Long-Term Hospitalized Patients: Insights from a Seven Year Experience. J. Steroid Biochem. Mol. Biol. 2019,189, 228-239. [CrossRef] [PubMed]
69. Deng, X.; Song, Y.; Manson, J.E.; Signorello, L.B.; Zhang, S.M.; Shrubsole, M.J.; Ness, R.M.; Seidner, D.L.; Dai, Q. Magnesium, Vitamin D Status and Mortality: Results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013,11,187. [CrossRef] [PubMed]
70. Coimbra, C.G.; (University of Sao Paulo, Sao Paulo, Brazil). Personal Communication. 2018. Recording of the lecture at the Congress of Human Medicine, Frankfurt, 14 April 2018. Available online: https://www.youtube.com/watch?v=w1XT0btvVSg (accessed on 31 January 2022).
71. Krafka, J., Jr. Simple Treatment for Psoriasis. J. Lab. Clin. Med. 1936,21,1147-1148.
72. Relhan, V.; Goel, K.; Kochhar, A.; Garg, V.; Wadhwa, B. Vitamin D and Skin Diseases: A Review. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 344. [CrossRef] [PubMed]
73. Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and Comorbid Diseases. J. Am. Acad. Dermatol. 2017, 76, 377-390. [CrossRef] [PubMed]
74. Gröber, U.; Spitz, J.; Reichrath, J.; Kisters, K.; Holick, M.F. Vitamin D. Dermato-Endocrinology 2013, 5, 331-347. [CrossRef]
75. Perez, A.; Raab, R.; Chen, T.C.; Turner, A.; Holick, M.F. Safety and Efficacy of Oral Calcitriol (1, 25 -Dihydroxyvitamin D3) for the Treatment of Psoriasis. Br. J. Dermatol. 1996,134,1070-1078. [CrossRef]
76. Morimoto, S.; Yoshikawa, K.; Kozoka, T.; Kitano, Y.; Imanaka, S.; Fukuo, K.; Koh, E.; Kumahara, Y. An Open Study of Vitamin D3 Treatment in Psoriasis Vulgaris. Br. J. Dermatol. 1986,115, 421-429. [CrossRef]
77. Smith, E.L.; Pincus, S.H.; Donovan, L.; Holick, M.F. A Novel Approach for the Evaluation and Treatment of Psoriasis. J. Am. Acad. Dermatol. 1988,19, 516-528. [CrossRef]
78. Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020,236,1-22. [CrossRef] [PubMed]
79. Upala, S.; Sanguankeo, A. Low 25-Hydroxyvitamin D Levels Are Associated with Vitiligo: A Systematic Review and MetaAnalysis. Photodermatol. Photoimmunol. Photomed. 2016, 32,181-190. [CrossRef] [PubMed]
80. Zhang, J.-Z.; Wang, M.; Ding, Y.; Gao, F.; Feng, Y.-Y.; Yakeya, B.; Wang, P.; Wu, X.-J.; Hu, F.-X.; Xian, J.; et al. Vitamin D Receptor Gene Polymorphism, Serum 25-Hydroxyvitamin D Levels, and Risk of Vitiligo. Medicine 2018, 97, e11506. [CrossRef] [PubMed]
81. Wang, J.; Thingholm, L.B.; Skieceviciene, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-Wide Association Analysis Identifies Variation in Vitamin D Receptor and Other Host Factors Influencing the Gut Microbiota. Nat. Genet. 2016, 48,1396-1406. [CrossRef]
82. Sipos, M.; Gerszi, D.; Dalloul, H.; Banyai, B.; Sziva, R.E.; Kollarics, R.; Magyar, P.; Török, M.; Äcs, N.; Szekeres, M.; et al. Vitamin D Deficiency and Gender Alter Vasoconstrictor and Vasodilator Reactivity in Rat Carotid Artery. Int. J. Mol. Sci. 2021, 22, 8029. [CrossRef]
83. Rogers, M.P.; Fozdar, M. Psychoneuroimmunology of autoimmune disorders. Adv. Neuroimmunol. 1996, 6,169-177. [CrossRef]
41+ VitaminDWiki pages with COIMBRA in title
This list is automatically updated
{LIST()}
67+ VitaminDWiki pages with HIGH-DOSE in title
This list is automatically updated
{LIST()}
VitaminDWiki - Multiple Sclerosis ( studies)
Multiple Sclerosis and (lots of) Vitamin D - book by patient on Coimbra protocol - Feb 2016
Multiple Sclerosis 32 percent less likely among those with more than 32 ng of vitamin D – Dec 2019
VitaminDWiki - Autoimmune category ( studies)
{include}
VitaminDWiki - Genetics category ( studies)
{include}
Vitamin D Receptor is associated in over 40 autoimmune studies
VitaminDWiki - Some diseases reduce vitamin D getting to blood or cells
{include}
