Vitamin D2 10X more bio-available when in grains of pollen
Spore exines increase vitamin D clinical bioavailability by mucoadhesion and bile triggered release
Journal of Controlled Release.Volume 350, October 2022, Pages 244-255,
https://doi.org/10.1016/j.jconrel.2022.08.017 PDF was purchased by VitaminDWiki
Alberto Diego-Taboada ab Thozhukat Sathyapalan c. FraserCourtsdMarkLorchaFarooqAlmutairieBenjamin P.BurkefKateHarrisgMartinKruusmägihThomasWaltherijJonathanBoothgAndrew N.BoaaStephen J.Archibaldf ColinThompsonk Stephen L.Atkinl Grahame Mackenzie ab g.mackenzie@hull.ac.uk
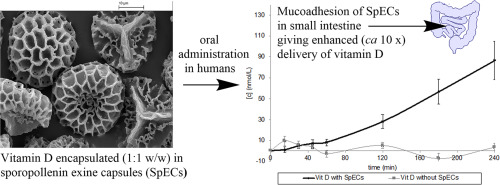
Image is from Google Image
Sporopollenin exine capsules (SpECs) are microcapsules derived from the outer shells (exines) of plant spore and pollen grains. This work reports the first clinical study on healthy volunteers to show enhanced bioavailability of vitamin D encapsulated in SpECs from Lycopodium clavatum L. spore grains vs vitamin D alone, and the first evidence (in vitro, ex vivo and in vivo) of mechanisms to account for the enhancement and release of the active in the small intestine.
Evidence for mucoadhesion of the SpECs contributing to the mechanism of the enhancement is based on:
(i) release profile over time of vitamin D in a double blind cross-over human study showing significant release in the small intestine;
(ii) in vivo particle counting data in rat showing preferred retention of SpECs vs synthetic beads;
(iii) ex vivo 99mTc labelling and counting data using rat small intestine sections showing preferred retention of SpECs vs synthetic beads;
(iv) in vitro mucoadhesion data.
Triggered release by bile in the small intestine was shown in vitro using solid state NMR and HPLC.
One of the authors wrote his PhD on this topic in 2015 (attached)
Abstract Sporopollenin is the polymeric fabric of the outer shell (known as exine) of plant spores and pollen grains. It is a highly cross-linked polymer composed of carbon, hydrogen and oxygen. It contains functional groups such as phenols, carboxylic acids and aliphatic alcohols as well as hydrophobic saturated and unsaturated alkyl chains. Sporopollenin is one of the most extraordinarily resistant polymers known in the organic world. It has the ability to resist harsh biological, chemical and physical environments, as intact sporopollenin exines have been found intact in some sedimentary rocks that are 500 million years old. This hydrophobic biopolymer has interesting properties, as its lipids (which are 20 % w/w of the total mass of extracted spores) possess liquid-like behavior in a dry solid polymer. This biopolymer has the ability to transform the liquid simple C18 fatty acids into "dry liquid" state when adsorbed or attached chemically to the surface. Sporopollenin exine capsules (SECs) have potential as a microparticle for vitamin and drug microencapsulation offering protection, controlled release and absorption enhancement. SECs have been shown to be able to encapsulate hydrophilic and hydrophobic drugs and vitamins and they have the ability to control the release of the encapsulated actives depending on the pH. In this study vitamin D, diclofenac sodium salt and mesalamine were encapsulated in L. clavatum SECs, and then released into buffer solutions at different pHs that mimic the GIT pHs. The release of such kind of vitamin and drugs showed the direct effect of pH factor on the release of the SECs contents. Vitamin D was released in pH 7.4, but was not in acidic pH. This was an interesting point, especially, for protecting the encapsulated vitamin D from the harsh acidic environment of stomach and release it in the mildly basic environment of small intestine. Also, diclofenac sodium was released fully in pH 7.4 within 12 hours, whereas less than 40 % was released in pH 1.5. However, the mesalamine showed a full release in acidic pH which showed that SECs need coating to be used for mesalamine. The adsorption of the drugs to the SECs was shown to be very low (0.4 %), which means that almost all the drug will be released and would not adsorb to the SECs surface, especially in the GIT when they would be flushed with fluid continuously. It is proposed that the ability of SECs to enhance the absorption is related to the mucoadhesivity that they possess, which was shown to be higher than chitosan and lower than carbopol. The mucoadhesion strength for L. clavatum powder polymer was statistically significant either with their lipids in (p-value = 0.03), or when their lipids were removed (p-value = 0.01).Also, the "dry liquid" C16 and C18 fatty acids of SECs might play role in absorption enhancement by disturbing the tight junction of the paracellular pathway transport, were most of the hydrophilic small molecules pass through to blood stream. The disintegration time of SECs tablets gives an idea about the best extraction protocol to use to increase the time of disintegration or decrease it depending in the site of absorption. For example, AH-SECs of L. clavatum was disintegrated within 2 h and lost 23.33 % of their mass, which is a preferable SECs type to be used for drug release in intestine within 2 h from the time of SECs tablet ingestion.
📄 Download the PDF from VitaminDWiki
References
[1] P. Borel, D. Caillaud, N.J. Cano, Vitamin d bioavailability: state of the art, Crit. Rev. Food Sci. Nutr. 55 (2015) 1193-1205, https://doi.org/10.1080/10408398.2012.688897
[2] V.M.M. Gimenez, F. Inserra, C.D. Tajer, J. Mariani, L. Ferder, R.J. Reiter, W. Manucha, Lungs as target of COVID-19 infection: protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment, Life Sci. 254 (2020), https://doi.org/10.1016/j.lfs.2020.11780
[3] G. Bertoldi, L. Gianesello, L.A. Calo, Letter: ACE2, Rho kinase inhibition and the potential role of vitamin D against COVID-19, Aliment. Pharmacol. Ther. 52 (577-578) (2020) 577-578, https://doi.org/10.1111/apt.15895
[4] F. Piedel, A. Rocka, M. Piwek, P.P. Jasielski, V. Petit, K. Rejdak, Correlation between vitamin D and alterations in MRI among patients with multiple sclerosis, Ann. Agricult. Environ. Med. 28 (2021) 372-377, https://doi.org/10.26444/aaem/127062
[5] J.S. Adams, M. Hewison, Update in Vitamin D, J. Clin. Endocrinol. Metab. 95 (2010) 471-478, https://doi.org/10.1210/jc.2009-1773
[6] R.L. Bailey, K.W. Dodd, J.A. Goldman, J.J. Gahche, J.T. Dwyer, A.J. Moshfegh, C.T. Sempos, M.F. Picciano, Estimation of total usual calcium and vitamin D intakes in the United States, J. Nutr., 140 817-822. https://doi.org/10.3945/jn.109.118539
[7] M. Kostecka, The role of healthy diet in the prevention of osteoporosis in perimenopausal period, Pakistan J. Med. Sci. 30 (2014) 763-768, https://doi.org/ 10.12669/pjms.304.4577
[8] J.P. Bilezikian, A.M. Formenti, R.A. Adler, N. Binkley, R. Bouillon, M. Lazaretti- Castro, C. Marcocci, N. Napoli, R. Rizzoli, A. Giustina, Vitamin D: dosing, levels, form, and route of administration: does one approach fit all? Rev. Endocr. Metab. Disord. 22 (2021) 1201-1218, https://doi.org/10.1007/s11154-021-09693-7 in VitaminDWiki
[9] A. Dwivedi, B. Gupta, S. Tiwari, D.D. Pratyush, S. Singh, S.K. Singh, Parenteral vitamin D supplementation is superior to oral in vitamin D insufficient patients with type 2 diabetes mellitus, Diabet. Metabol. Syndrome-Clin. Res. Rev. 11 (2017) S373-S375, https://doi.org/10.1016/j.dsx.2017.03.019
[10] E.A. Argao, J.E. Heubi, B.W. Hollis, R.C. Tsang, D-alpha-Tocopheryl polyethylene glycol-1000 succinate enhances the absorption of vitamin-d in chronic cholestatic liver-disease of infancy and childhood, Pediatr. Res. 31 (1992) 146-150, https:// doi.org/10.1203/00006450-199202000-00011
[11] J. Szejtli, E. Bollapusztai, M. Tardylengyel, P. Szabo, T. Ferenczy, Preparation, properties and biological-activity of beta-cyclodextrin inclusion complex of menadione, Pharmazie 38 (1983) 189-193
[12] E. Semo, E. Kesselman, D. Danino, Y.D. Livney, Casein micelle as a natural nano- capsular vehicle for nutraceuticals, Food Hydrocoll. 21 (2007) 936-942, https:// doi.org/10.1016/j.foodhyd.2006.09.006
[13] M. Haham, S. Ish-Shalom, M. Nodelman, I. Duek, E. Segal, M. Kustanovich, Y. D. Livney, Stability and bioavailability of vitamin D nanoencapsulated in casein micelles, Food Funct. 3 (2012) 737-744, https://doi.org/10.1039/c2fo10249h
[14] E. Simoliunas, I. Rinkunaite, Z. Bukelskiene, V. Bukelskiene, Bioavailability of different vitamin D oral supplements in laboratory animal model, Medicina- Lithuania 55 (2019), https://doi.org/10.3390/medicina55060265
[15] A. Wakil, G. Mackenzie, A. Diego-Taboada, J.G. Bell, S.L. Atkin, Enhanced bioavailability of eicosapentaenoic acid from fish oil after encapsulation within plant spore exines as microcapsules, Lipids 45 (2010) 645-649, https://doi.org/ 10.1007/s11745-010-3427-y
[16] H. Bouwmeester, S. Dekkers, M.Y. Noordam, W.I. Hagens, A.S. Bulder, C. de Heer, S.E. ten Voorde, S.W. Wijnhoven, H.J. Marvin, A.J. Sips, Review of health safety aspects of nanotechnologies in food production, Regul. Toxicol. Pharmacol. 53 (2009) 52-62, https://doi.org/10.10Wj.yrtph.2008.10.008
[17] G. Shaw, Sporo pollen in, in: J.B. Harborne (Ed.), Phytochemical Phylogeny, London & New York, Academic Press, 1970, pp. 31-35
[18] A. Mikhael, K. Jurcic, C. Schneider, D. Karr, G.L. Fisher, T.D. Fridgen, A. Diego- Taboada, P.E. Georghiou, G. Mackenzie, J. Banoub, Demystifying and unravelling the molecular structure of the biopolymer sporo pollen in, Rapid Commun. Mass. Sp 34 (2020), https://doi.org/10.1002/rcm.8740
[19] A. Diego-Taboada, L. Maillet, J.H. Banoub, M. Lorch, A.S. Rigby, A.N. Boa, S. L. Atkin, G. Mackenzie, Protein free microcapsules obtained from plant spores as a model for drug delivery: ibuprofen encapsulation, release and taste masking,
J. Mater. Chem. B 1 (2013) 707-713, https://doi.org/10.1039/c2tb00228k. S. Gubatz, S. Herminghaus, B. Meurer, D. Strack, R. Wiermann, The location of hydroxycinnamic acid amides in the exine of Corylus pollen , pollen Spores 28 (1986) 347-354
[20] K. Schulze Osthoff, R. Wiermann, Phenols as integrated compounds of sporo pollen in from Pinus pollen , J. Plant Physiol. 131 (1987) 5-15, https://doi. org/10.1016/S0176-1617(87)80262-6
[21] S. Herminghaus, S. Gubatz, S. Arendt, R. Wiermann, The occurrence of phenols as degradation products of natural sporo pollen in - A comparison with synthetic sporo pollen in, Zeitschrift Für Naturforschung C-a, J. Biosci. 43 (1988) 491-500, https://doi.org/10.1515/znc-1988-7-803
[22] S. Wilmesmeier, S. Steuernagel, R. Wiermann, Comparative Ftir and C-13 Cp/mas Nmr spectroscopic investigations on sporo pollen in of different systematic origins, Zeitschrift Fur Naturforschung C-a, J. Biosci. 48 (1993) 697-701, https://doi.org/10.1515/znc-1993-9-1003
[23] T.F. Fan, M.G. Potroz, E.L. Tan, M.S. Ibrahim, E. Miyako, N.J. Cho, Species-specific biodegradation of sporo pollen in-based microcapsules, Sci. Rep. 9 (2019), https://doi.org/10.1038/s41598-019-46131-w
[24] T.F. Fan, M.G. Potroz, E.L. Tan, J.H. Park, E. Miyako, N.J. Cho, Human blood plasma catalyses the degradation of Lycopodium plant sporoderm microcapsules, Sci. Rep. 9 (2019), https://doi.org/10.1038/s41598-019-39858-z
[25] M. Lorch, M.J. Thomasson, A. Diego-Taboada, S. Barrier, S.L. Atkin, G. Mackenzie, S. J. Archibald, MRI contrast agent delivery using spore capsules: controlled release in blood plasma, Chem. Commun. (2009) 6442-6444, https://doi.org/10.1039/ b909551a
[26] J. Wittborn, K.V. Rao, G. El-Ghazaly, J.R. Rowley, Nanoscale similarities in the substructure of the exines of Fagus pollen grains and Lycopodium spores, Ann. Bot. 82 (1998) 141-145, https://doi.org/10.1006/anbo.1998.0649
[27] S. Barrier, A. Diego-Taboada, M.J. Thomasson, L. Madden, J.C. Pointon, J. D. Wadhawan, S.T. Beckett, S.L. Atkin, G. Mackenzie, Viability of plant spore exine capsules for microencapsulation, J. Mater. Chem. 21 (2011) 975-981
[28] S. Barrier, A.S. Rigby, A. Diego-Taboada, M.J. Thomasson, G. Mackenzie, S. L. Atkin, Sporo pollen in exines: a novel natural taste masking material, Lwt-Food Sci. Technol. 43 (2010) 73-76, https://doi.org/10.10Wj.lwt.2009.07.001
[29] A. Diego-Taboada, P. Cousson, E. Raynaud, Y. Huang, M. Lorch, B.P. Binks, Y. Queneau, A.N. Boa, S.L. Atkin, S.T. Beckett, G. Mackenzie, Sequestration of edible oil from emulsions using new single and double layered microcapsules from plant spores, J. Mater. Chem. 22 (2012) 9767-9773, https://doi.org/10.1039/ c2jm00103a
[30] I. Sargin, L. Akyuz, M. Kaya, K. Yildirim, S. Ertosun, G.H. Aydin, M. Topal, G. Tan, T. Ceter, Controlled release and anti-proliferative effect of imatinib mesylate loaded sporo pollen in microcapsules extracted from pollen s of Betula pendula, Int. J. Biol. Macromol. (2017), https://doi.org/10.1016Zj.ijbiomac.2017.07.093
[31] A.K. Prabhakar, H.Y. Lai, M.G. Potroz, M.K. Corliss, J.H. Park, R.C. Mundargi, D. Cho, S.I. Bang, N.J. Cho, Chemical processing strategies to obtain sporo pollen in exine capsules from multi-compartmental pine pollen , J. Ind. Eng. Chem. 53 (2017) 375-385, https://doi.org/10.10Wj.jiec.2017.05.009
[32] M. Keles, Preparation of heterogeneous palladium catalysts supported on sporo pollen in for heck coupling reactions, synthesis and reactivity in inorganic metal-organic and nano-metal, Chemistry 43 (2013) 575-579, https://doi.org/10.1080/15533174.2012.749895
[33] M. Mujtaba, I. Sargin, L. Akyuz, T. Ceter, M. Kaya, Newly isolated sporo pollen in microcages from Platanus orientalis pollen s as a vehicle for controlled drug delivery, Mater. Sci. Eng. C-Mater. Biol. Appl. 77 (2017) 263-270, https://doi.org/ 10.1016/j.msec.2016.11.009
[34] L. Akyuz, I. Sargin, M. Kaya, T. Ceter, I. Akata, A new pollen -derived microcarrier for pantoprazole delivery, Mater. Sci. Eng. C-Mater. Biol. Appl. 71 (2017) 937-942, https://doi.org/10.1016/j.msec.2017.02.176
[35] S.M. Alshehri, H.A. Al-Lohedan, A.A. Chaudhary, E. Al-Farraj, N. Alhokbany, Z. Issa, S. Alhousine, T. Ahamad, Delivery of ibuprofen by natural macroporous sporo pollen in exine capsules extracted from Phoenix dactylifera L, Eur. J. Pharm. Sci. 88 (2016) 158-165
[36] S.M. Alshehri, H.A. Al-Lohedan, E. Al-Farraj, N. Alhokbany, A.A. Chaudhary, T. Ahamad, Macroporous natural capsules extracted from Phoenix dactylifera L. spore and their application in oral drugs delivery, Int. J. Pharm. 504 (2016) 39-47, https://doi.org/10.1016/j.ijpharm.2016.02.049
[37] M.J. Uddin, H.S. Gill, Ragweed pollen as an oral vaccine delivery system: mechanistic insights, J. Control. Release 268 (2017) 416-426, https://doi.org/ 10.1016/j.jconrel.2017.10.019
[38] N. Sudareva, O. Suvorova, N. Saprykina, A. Vilesov, P. Bel’tiukov, S. Petunov, A. Radilov, Two-level delivery systems for oral administration of peptides and A. Paharia, A.K. Yadav, G. Rai, S.K. Jain, S.S. Pancholi, G.P. Agrawal, Eudragit- coated pectin microspheres of 5-fluorouracil for colon targeting, AAPS PharmSciTech 8 (2007), https://doi.org/10.1208/pt0801012
[39] R.C. Mundargi, M.G. Potroz, S. Park, J.H. Park, H. Shirahama, J.H. Lee, J. Seo, N. J. Cho, Lycopodium spores: a naturally manufactured, superrobust biomaterial for drug delivery, Adv. Funct. Mater. 26 (2016) 487-497, https://doi.org/10.1002/adfm.201502322
[40] S.V. Lale, H.S. Gill, pollen grains as a novel microcarrier for oral delivery of proteins, Int. J. Pharm. 552 (2018) 352-359, https://doi.org/10.1016/_j. ijpharm.2018.10.016
[41] M.G. Potroz, R.C. Mundargi, J.J. Gillissen, E.L. Tan, S. Meker, J.H. Park, H. Jung, S. Park, D. Cho, S.I. Bang, N.J. Cho, Plant-based hollow microcapsules for oral delivery applications: toward 10.1002/adfm.2017002700ptimized loading and controlled release, Adv. Funct. Mater. 27 (2017)
[42] T.L. Harris, C.J. Wenthur, A. Diego-Taboada, G. Mackenzie, T.S. Corbitt, K. D. Janda, Lycopodium clavatum exine microcapsules enable safe oral delivery of 3,4-diaminopyridine for treatment of botulinum neurotoxin a intoxication, Chem. Commun. 52 (2016) 4187-4190, https://doi.org/10.1039/c6cc00615a
[43] N.M. Meligi, A.K.F. Dyab, V.N. Paunov, Sustained in vitro and in vivo delivery of metformin from plant pollen -derived composite microcapsules, Pharmaceutics 13 (2021), https://doi.org/10.3390/pharmaceutics13071048
[44] S. Barrier, A.S. Rigby, A. Diego-Taboada, M.J. Thomasson, G. Mackenzie, S.L. Atkin, Sporo pollen in exines: a novel natural taste masking material, LWT Food Sci. Technol. 43 (2010) 73-76, https://doi.org/10.10Wj.lwt.2009.07.001
[45] G. Erdtman, The acetolysis method. A revised description, Sven. Bot. Tidskr. 54 (1960) 561-564. S. Barrier, A. Lobbert, A.J. Boasman, A.N. Boa, M. Lorch, S.L. Atkin, G. Mackenzie, Access to a primary aminosporo pollen in solid support from plant spores, Green Chem. 12 (2010) 234-240, https://doi.org/10.1039/B913215e
[46] R. Shaikh, T.R. Raj Singh, M.J. Garland, A.D. Woolfson, R.F. Donnelly, Mucoadhesive drug delivery systems, J. Pharmacy Bioallied Sci. 3 (2011) 89-100
[47] V.V. Khutoryanskiy, Advances in mucoadhesion and mucoadhesive polymers, Macromol. Biosci. 11 (2011) 748-764, https://doi.org/10.1002/mabi.201000388
[48] B.P. Binks, A.N. Boa, M.A. Kibble, G. Mackenzie, A. Rocher, Sporo pollen in capsules at fluid interfaces: particle-stabilised emulsions and liquid marbles, Soft Matter 7 (2011), 4017-4024.v
[49] M.J. Thomasson, D.J. Baldwin, A. Diego-Taboada, S.L. Atkin, G. Mackenzie, J. D. Wadhawan, Electrochemistry and charge transport in sporo pollen in particle arrays, Electrochem. Commun. 12 (2010) 1428-1431, https://doi.org/10.1016/j. elecom.2010.07.038
[50] A. Diego-Taboada, S.T. Beckett, S.L. Atkin, G. Mackenzie, Hollow pollen shells to enhance drug delivery, Pharmaceutics 6 (2014) 80-96, https://doi.org/10.3390/ pharmaceutics6010080
[51] J. Brooks, G. Shaw, Sporo pollen in a review of its chemistry paleochemistry and geochemistry, Grana 17 (1978) 91-98, https://doi.org/10.1080/ 00173137809428858
[52] F.S. Li, P. Phyo, J. Jacobowitz, M. Hong, J.K. Weng, The molecular structure of plant sporo pollen in, Nat. Plants 5 (2019) 41-46, https://doi.org/10.1038/s41477- 018-0330-7
[53] D.C. Mildenhall, P.E.J. Wiltshire, V.M. Bryant, Forensic palynology: why do it and how it works, Forensic Sci. Int. 163 (2006) 163-172, https://doi.org/10.1016/j. forsciint.2006.07.012
[54] M.G. Simpson, pollen and spores - patterns of diversification - Blackmore,S, Barnes, SH, Science 259 (1993) 702, https://doi.org/10.1126/science.259.5095.702
[55] S. Blackmore, pollen and spores - patterns of diversification, Evol. Trends Plants 4 (1990) 78-79
[56] I.J. Gomez, W.B. Goodwin, D. Sabo, Z.J. Zhang, K.H. Sandhage, J.C. Meredith, Three-dimensional magnetite replicas of pollen particles with tailorable and predictable multimodal adhesion, J. Mater. Chem. C 3 (2015) 632-643, https:// doi.org/10.1039/c4tc01938e
[57] W.B. Goodwin, I.J. Gomez, Y. Fang, J.C. Meredith, K.H. Sandhage, Conversion of pollen particles into three-dimensional ceramic replicas tailored for multimodal adhesion, Chem. Mater. 25 (2013) 4529-4536, https://doi.org/10.1021/ cm402226w
[58] J. Urbanczyk, M.A. Fernandez Casado, T.E. Diaz, P. Heras, M. Infante, A.G. Borrego, Spectral fluorescence variation of pollen and spores from recent peat forming plants, Int. J. Coal Geol. 131 (2014) 263-273, https://doi.org/10.1016/j. coal.2014.06.024
[59] K. Holt, G. Allen, R. Hodgson, S. Marsland, J. Flenley, Progress towards an automated trainable pollen location and classifier system for use in the palynology laboratory, Rev. Palaeobot. Palynol. 167 (2011) 175-183
[60] H.F. Linskens, W. Jorde, Persorption of lycopodium spores and pollen grains, Naturwissenschaften 61 (1974) 275-276, https://doi.org/10.1016/j. revpalbo.2011.08.006
[61] P.G. Jenkins, K.A. Howard, N.W. Blackhall, N.W. Thomas, S.S. Davis, D.T. Ohagan, Microparticulate absorption from the rat intestine, J. Control. Release 29 (1994) 339-350, https://doi.org/10.1016/0168-3659(94)90079-5
[62] S.U. Atwe, Y. Ma, H.S. Gill, pollen grains for oral vaccination, J. Control. Release 194 (2014) 45-52, https://doi.org/10.10Wj.jconrel.2014.08.010
[63] P. Chen, P.A. Eggleston, Allergenic proteins are fragmented in low concentrations of sodium hypochlorite, Clin. Exp. Allergy 31 (2001) 1086-1093, https://doi.org/ 10.1046/j.1365-2222.2001.01127.x.
VitaminDWiki - Vitamin D3 instead of D2 category contains
{include}
Sent email - asking if they have considered VitaminD3: no response
8 citations of study as of June 2024
- Extraordinary microcarriers derived from spores and pollens - Dec 2022 PDF behind $50 paywall
6+ VitaminDWiki pages have BIO-AVAILABLE in the title
This list is automatically updated
{LIST()}
