EUA quickly written for one COVID treatment, EUA might be possible for Vitamin D
FDA requires very little documentation and can quickly decide in emergencies such as COVID
The principal investigator wrote an application for Emergency Use of Fluvoxamine for SARS-COV-2 in 4 days
VitaminDWiki has requested a copy of his application and tips on how to communicate with the FDA
--The FDA gave the EUA about a week later-- I had misread the WSJ article, it has not been approved (yet)
It appears that EUAs must specify:
Dose size,
Dosing interval
When it is taken (before or after entering the hospital, for example)
How it is taken: Oral, injection, topical
The FDA gives EUAs for Food, Drugs, Chemicals or Devices - but not supplements
Perhaps the FDA could give a EUA for Vitamin D (Which has been used for food fortification)
The FDA could also issue a EUA for semi-activated Vitamin D = Calcidiol = Calcifediol =25(OH)D
which is a prescribed (high profit) drug in the US
Reported by: 📄 Wall St Journal PDF 📄 Trial Site News PDF 📄 Medpage PDF
📄 FDA EUA Guidelines FDA EUA page
Ranking of Early Treatments of COVID

Note: Vitamin D appears to be even better for prophylaxis (prevention) than early treatment
but there is a lot more data for early treatment
Note: It appears that high-dose Vitamin D is the only way to quickly improve the immune system
Fluvoxamine

-
- Decreased interest in sexual intercourse, inability to have or keep an erection. loss in sexual ability, desire, drive, or performance, constipation, headache, trouble sleeping. unusual tiredness
VitaminDWiki - Calcifediol and Virus studies (7 as of Dec 2021)
{category}
VitaminDWiki - some Calcifediol studies (5 as of Dec 2021)
Calcifediol (Calcidiol) is far less cost-effective and available than Vitamin D – RCT June 2021
Weekly response to semi-activated vitamin D slightly better than standard – RCT Nov 2019
VitaminDWiki - COVID-19 treated by Vitamin D - studies, reports, videos
{include}
Summary of data of Vitamin D fighting COVID (2 forms)
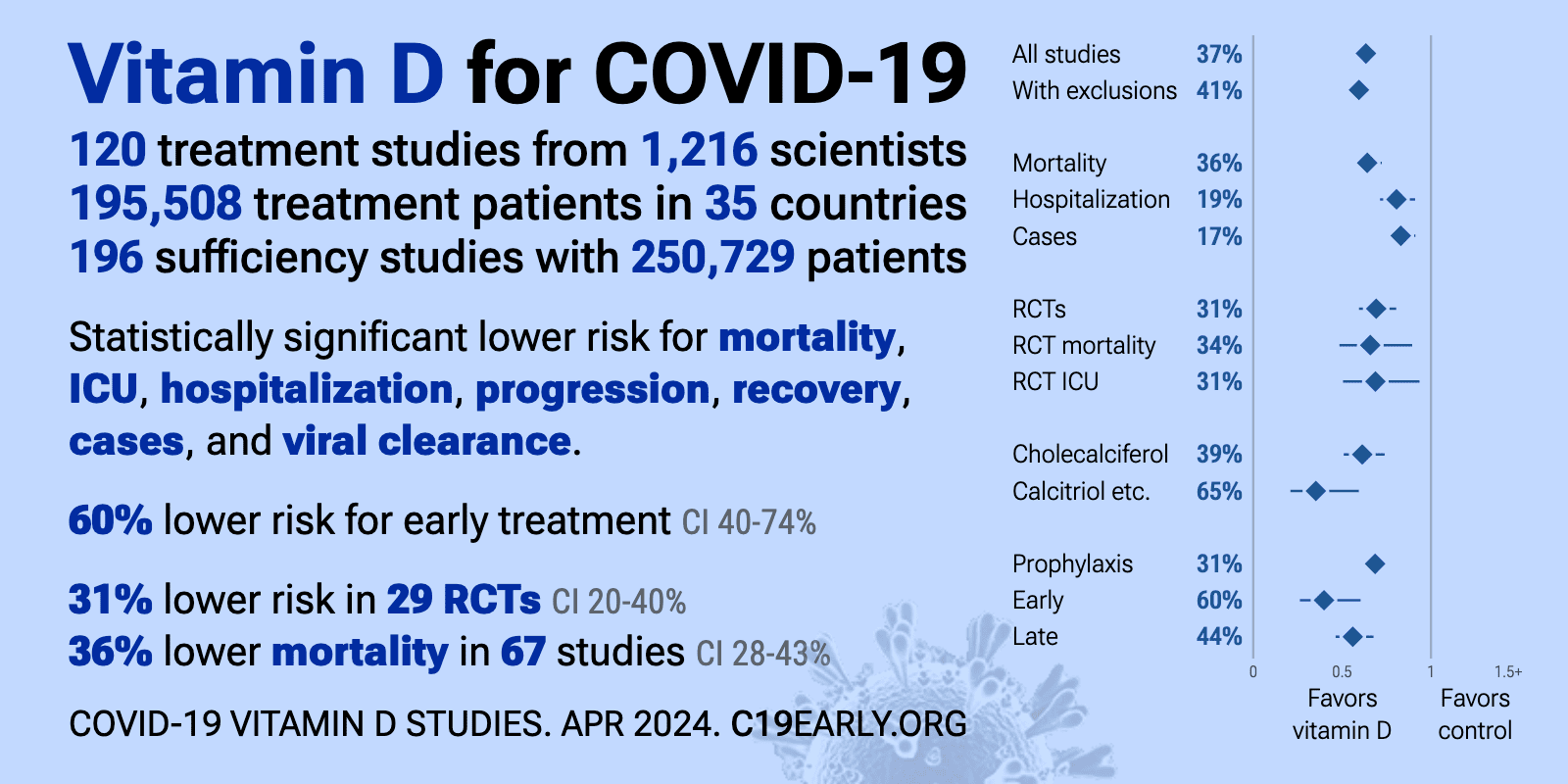
- The above image is automatically updated
