Vitamin D3 vs serum D3 (Calcitriol, HyD)
Junk
Oral supplementation with 25(OH)D3 versus vitamin D3: Effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity
Journal of Bone and Mineral Research, Volume 27, Issue 1, pages 160–169, January 2012
Heike Annette Bischoff-Ferrari1,2,*, Bess Dawson-Hughes3, Elisabeth Stöcklin4, Eduard Sidelnikov1, Walter Churchill Willett5, John Orav Edel6, Hannes Balthasar Stähelin7, Swen Wolfram4, Alexander Jetter8, Joseph Schwager4, Jana Henschkowski1, Arnold von Eckardstein9, Andreas Egli1
1 Centre on Aging and Mobility, University of Zurich and Waid City Hospital Zurich, Zurich, Switzerland
2 Department of Rheumatology, University Hospital Zurich, Zurich, Switzerland
3 USDA Human Nutrition Research Center on Aging, Tufts University, Boston, USA
4 Research and Development, DNP Nutritional Products, Basel, Switzerland
5 Department of Nutrition, Harvard School of Public Health, Boston, MA, USA
6 Department of Biostatistics, Harvard School of Public Health, Boston, MA, USA
7 Department of Geriatrics, University Hospital Basel, Basel Switzerland
8 Department of Clinical Pharmacology and Toxicology, University of Zurich, Zurich, Switzerland
9 Institute of Clinical Chemistry, University of Zurich, Zurich, Switzerland
Email: Heike Annette Bischoff-Ferrari (HeikeABischoff@aol.com)
- Centre on Aging and Mobility, University Hospital Zurich, Gloriastrasse 25, 8091 Zurich, Switzerland.
Article first published online: 22 DEC 2011
Copyright © 2012 American Society for Bone and Mineral Research
PDF is attached at the bottom of this page
Abstract
To test the effect of 25(OH)D3 (HyD) compared to vitamin D3 on serum 25-hydroxyvitamin D levels (25(OH)D), lower extremity function, blood pressure, and markers of innate immunity. Twenty healthy postmenopausal women with an average 25(OH)D level of 13.2±3.9ng/mL (mean±SD) and a mean age of 61.5±7.2 years were randomized to either 20µg of HyD or 20µg (800IU) of vitamin D3 per day in a double-blind manner. We measured on 14 visits over 4 months, 25(OH)D serum levels, blood pressure, and seven markers of innate immunity (eotaxin, interleukin [IL]-8, IL-12, interferon gamma-induced protein 10kDa [IP-10], monocyte chemotactic protein-1 [MCP-1], macrophage inflammatory protein beta [MIP-1], and “Regulated upon Activation, Normal T-cell Expressed, and Secreted” [RANTES]). At baseline and at 4 months, a test battery for lower extremity function (knee extensor and flexor strength, timed up and go, repeated sit-to-stand) was assessed. All analyses were adjusted for baseline measurement, age, and body mass index. Mean 25(OH)D levels increased to 69.5ng/mL in the HyD group. This rise was immediate and sustained. Mean 25(OH)D levels increased to 31.0ng/mL with a slow increase in the vitamin D3 group. Women on HyD compared with vitamin D3 had a 2.8-fold increased odds of maintained or improved lower extremity function (odds ratio [OR]=2.79; 95% confidence interval [CI], 1.18–6.58), and a 5.7-mmHg decrease in systolic blood pressure (p=0.0002). Both types of vitamin D contributed to a decrease in five out of seven markers of innate immunity, significantly more pronounced with HyD for eotaxin, IL-12, MCP-1, and MIP-1 . There were no cases of hypercalcemia at any time point. Twenty micrograms (20µg) of HyD per day resulted in a safe, immediate, and sustained increase in 25(OH)D serum levels in all participants, which may explain its significant benefit on lower extremity function, systolic blood pressure, and innate immune response compared with vitamin D3.
© 2012 American Society for Bone and Mineral Research
Introduction
Desirable thresholds for 25(OH)D have been discussed recently, both for bone health and non-skeletal endpoints. Although the 2010 Institute of Medicine (IOM) recommendations support a 25(OH)D threshold of 20ng/mL for bone health,1 the International Osteoporosis Foundation, in their 2010 position paper on vitamin D, recommends a threshold of 30ng/mL for optimal fall and fracture reduction,2 consistent with the most recent recommendations by the U.S. Endocrine Society published in June 2011.3 The recommendations by the International Osteoporosis Foundation (IOF) and the Endocrine Society are based on two meta-analyses of double-blind randomized controlled trials that suggested fall reduction required a threshold of at least 24ng/mL4 and fracture reduction a threshold of at least 30ng/mL.5
Regarding general health endpoints, the Endocrine Society states that while evidence from randomized controlled trials is lacking, numerous epidemiological studies have suggested that a 25(OH)D blood level of 30ng/mL and above may have additional health benefits in reducing the risk of common cancers, autoimmune diseases, type 2 diabetes, cardiovascular disease, and infectious diseases.6–10 In contrast, the IOM concludes that there is no evidence that a 25(OH)D threshold greater than 20ng/mL has any benefit to health.11
Although no consensus has been achieved as to whether 20ng/mL or 30ng/mL is the optimal threshold of vitamin D, as reflected in these most recent recommendations, most agree that many people are vitamin D–deficient and need vitamin D supplements to meet their vitamin D requirement.12 The most common form of dietary supplementation used today is cholecalciferol or vitamin D3 and most healthy adults reach a target range of 20ng/mL with 600 to 800IU vitamin D per day. However, the target range of at least 30ng/mL may require 1800IU to 4000IU vitamin D3 per day.13
As an alternative strategy to enhance 25(OH)D levels, supplementation with the 25(OH)D3 metabolite itself (HyD) has been suggested. Compared to vitamin D3, HyD has hydrophilic properties, has a much shorter half-life of 8 to 11hours after oral administration, and causes a rapid increase in serum 25(OH)D levels.14, 15 In earlier studies, oral intake of HyD resulted in a potent increase in serum 25(OH)D levels and parathyroid hormone suppression.16–21 Further, some smaller trials reported a benefit on bone density among groups of cardiac and kidney transplant patients, and elderly hip fracture patients19, 22, 23; although this was not confirmed in a larger trial among 438 seniors.18 However, the treatment dose of HyD in the larger trial was low with 15µg per day.18 Finally, in laboratory fracture-healing studies, HyD improved mechanical strength of the fractured bone in old rats.23
The goal of this study was to assess the efficacy of the 25(OH)D3 metabolite (HyD) compared to vitamin D3 in increasing 25(OH)D serum levels. Further, we aimed to investigate the effects of HyD compared to vitamin D3 on blood pressure, lower extremity function, and markers of innate immunity.
Patients and Methods
Participant population
For the study, we recruited 20 white postmenopausal women 50 to 70 years of age, in general good health. Other inclusion criteria were serum 25(OH)D level between 8 to 24ng/mL, body mass index between 18 and 29kg/m2, and ability of giving informed consent. Exclusion criteria were composed of medical contraindications to vitamin D supplements, medical conditions, or medication use that would alter pharmacokinetics of study products or otherwise interfere with the study. Specific exclusion criteria are listed in Appendix 1. All subjects were recruited at the Centre on Aging and Mobility, University Hospital Zurich, Zurich, Switzerland. This study was approved by the Zurich Cantonal Ethical Committee and Swiss Medic. Written informed consent was obtained from all study participants. The trial has been registered at the international trial registry (NCT00718276).
Clinical trial protocol
Age-eligible women, who were willing to participate, were invited for a screening visit at which demographic characteristics were collected, health status and habits were evaluated against inclusion/exclusion criteria, and fasting blood was drawn to assess serum levels of 25(OH)D, calcium, creatinine, and albumin, and glucose levels. Women who passed eligibility criteria signed informed consent and were randomly assigned to one of four groups: 20µg HyD daily, 20µg (800IU) vitamin D3 daily, 140µg HyD weekly, and 140µg (5600IU) vitamin D3 weekly. All supplements were taken orally. We randomized five participants to each treatment group. The dose of vitamin D3 was chosen to compare to the current standard for vitamin D3(20µg=800IU/d). The same dose was chosen for HyD, which, based on our review of the literature, offered an optimal balance of efficacy and safety.16, 21 We randomized 15 additional women to one-time bolus groups, which were only included in a pharmacokinetic analysis, while clinical endpoint evaluations for this manuscript are restricted to the daily and weekly treatment groups (n=20). As the results between daily and weekly HyD and vitamin D3 supplementation did not differ significantly, the daily and weekly groups for each supplementation were combined for the analysis.
DSM Nutritional Products, Ltd., Switzerland, manufactured crystalline 25(OH)D3 (HyD®) and vitamin D3 and formulated both as identical spray-dried powders stabilized with DL-alpha tocopherol. Study capsules (hard gelatin capsules) were provided by DSM Nutritional Products, Ltd., Switzerland, and contained HyD or cholecalciferol (vitamin D3), respectively. Subjects, investigators, study physician, and nurses were aware of the treatment regimen (daily or weekly), but blinded with regard to the type of treatment (HyD or vitamin D3). The physiotherapist who assessed all functional endpoints was blinded to both regimen and type of treatment.
Clinical visits
Participants attended one screening visit and 14 clinical visits during a 4-month trial period. During the first week of the study, participants attended five daily visits. The baseline visit (visit 2) included 24-hour pharmacokinetic profiling for HyD and vitamin D3, with repeated measurements of serum levels of calcium, creatinine, and 25(OH)D. Additionally, a baseline level of intact parathyroid hormone (PTH), albumin, insulin, fasting glucose, 1,25(OH)2D, and immunity markers (eotaxin, interleukin [IL]-8, IL-12, interferon gamma-induced protein 10kDa [IP-10], monocyte chemotactic protein-1 [MCP-1], macrophage inflammatory protein beta [MIP-1], “Regulated upon Activation, Normal T-cell Expressed, and Secreted” [RANTES]) were assessed. Further, all subjects underwent functional testing of lower extremities, including validated and standardized procedures for knee flexor/extensor strength, timed up-and-go test, and repeated sit-to-stand test.24 The remaining four daily visits of the first week (visits 3–6) included measurements of circulating 25(OH)D, immunity markers, albumin, serum calcium, and creatinine.
The following eight biweekly visits (visits 7–14) included the same measurements as visits 3 to 6; however, some visits included additional measurements. PTH was measured at visits 8, 10, and 12 (weeks 3, 7, and 11, respectively); visit 10 (week 7) included measurements of fasting glucose and insulin. In addition to the first clinical visit, 1,25(OH)2D was measured two more times during the follow-up period: on visits 9 and 14 (weeks 8 and 17, respectively). During week 16, participants underwent two final clinical visits. Visit 14 included 24-hour pharmacokinetic profiling and extended set of measurements identical to that for visit 2 (baseline visit).
Biomarker assays
We assessed serum 25(OH)D concentration by a sensitive and selective assay based on liquid chromatography coupled to tandem mass spectrometry detection (HPLC-MS/MS), which selectively measures 25(OH)D3 and was validated in an international NIST comparison (www.nist.gov; see Supporting Information for detailed methods or contact the authors). 1,25-hydroxyvitamin D was assessed by a radioimmunoassay. Intact PTH was measured in EDTA blood by means of an electrochemiluminescence immune assay.25 Insulin levels were measured by a chemiluminescence assay.26 Multiparametric kits for cytokine and chemokine determinations were purchased from BIO-RAD Laboratories (Hercules, CA, USA) and used in the LiquiChip Workstation IS 200 (Qiagen, Hilden, Germany) according to the manufacturers' instructions. Measurements included: serum concentration for IP-10, eotaxin, IL-8, IL-12, MCP-1, MIP-1, and RANTES.
Compliance and safety
We assessed adherence to daily and weekly treatment regimen by capsule count at each study visit. Those on the weekly treatment regimen took one-half of their capsules at biweekly visits under a nurse's supervision. In addition, adherence was assessed by measuring serum concentrations of 25(OH)D at the end of the study.
Adverse events were monitored by interview during each study visit. Serum calcium levels and urinary calcium excretion (calcium/creatinine ratio in spot urine) were assessed at each visit and on the two pharmacokinetic days at each time point.
Statistical analysis
The statistical analysis of clinical outcomes is focused on women who were on daily or weekly HyD or vitamin D3 treatment (n=20) and was based on intent to treat. Results of exploratory analyses showed that the effects of daily and weekly treatment regimens with either HyD or vitamin D3 were not significantly different. Based on that, the original four groups were consolidated into two groups: those treated with HyD and those treated with vitamin D3. Each group included 10 participants. Comparability of treatment groups with respect to important demographic characteristics, lower extremity function indicators, and plasma levels of immunity markers at baseline were assessed by one-way ANOVA.
We used generalized estimation equations (GEE) models to compare the odds of maintaining or improving lower extremity function during the 4-month follow-up between the HyD and vitamin D3 treatment groups. For these models, a dichotomous variable was created indicating those whose results worsened versus those whose results improved or stayed the same for the duration of the follow-up period. Amount of change in lower extremity function was assessed by linear models comparing the results of functional tests (knee extensor and flexor strength, the timed up-and-go test, and the repeated sit-to-stand test) between the groups at the end of follow-up. The models controlled for the results of the same tests at baseline, age, and body mass index (BMI). Parameters that were assessed at multiple clinical visits (systolic and diastolic blood pressure, plasma levels of 25(OH)D and immunity markers) were analyzed by linear mixed models.
Results
Table 1 shows baseline characteristics of the study population. Groups randomized for treatment with vitamin D3 and HyD were comparable to each other with respect to all investigated characteristics. Participants randomized to the vitamin D3 group tended to be older than those in HyD group (63.5 versus 59.5 years of age), had higher BMI (25.5 versus 23.2kg/m2), and higher average blood pressure (126/75 versus 119/70mmHg); average blood levels of insulin and PTH were somewhat lower than in the HyD group. None of these differences, however, were statistically significant (Table 1).
Table 1. Baseline Characteristics of the Study Population
Characteristic Vitamin D3 (n=10) HyD (n=10) p
Mean SD Mean SD
Values are mean±SD; values of p are based on t test for normally distributed variables and on Wilcoxon rank-sum test for those that were not normally distributed.
HyD=25(OH)D3; BMI=body mass index; IL=interleukin; IP-10=interferon gamma-induced protein 10kDa; MCP-1=monocyte chemotactic protein-1; MIP-1=macrophage inflammatory protein beta; RANTES=Regulated upon Activation, Normal T-cell Expressed, and Secreted.
Anthropometrics
Age (years) 63.45 7.78 59.48 6.27 0.22
BMI (kg/m2) 25.49 3.38 23.24 3.22 0.15
Functional tests
Systolic blood pressure (mmHg) 126.30 14.27 118.70 10.90 0.20
Diastolic blood pressure (mmHg) 75.30 8.19 70.00 5.56 0.11
Knee flexion strength (N) 180.64 34.99 202.57 29.89 0.15
Knee extension strength (N) 292.40 41.85 286.92 76.37 0.84
Timed up-and-go test (second) 7.44 1.34 7.48 0.54 0.93
Repeated sit-to-stand test (second) 8.34 2.41 7.24 1.57 0.24
Blood plasma/serum levels
25(OH)D (ng/mL) 14.18 3.61 12.28 4.08 0.28
1,25(OH)2D (pg/mL) 38.61 12.10 33.02 13.63 0.36
Serum calcium (mmol/L) 2.27 0.08 2.26 0.07 0.71
Parathyroid hormone (pg/mL) 54.87 10.71 63.22 16.37 0.19
Insulin (mIU/L) 2.01 2.09 5.59 5.92 0.33
Serum glucose (mmol/L) 5.33 0.43 5.28 0.61 0.83
Eotaxin plasma level (pg/mL) 27.48 8.97 34.01 13.78 0.23
IL-8 (pg/mL) 2.90 0.84 3.04 0.68 0.68
IL-12 (pg/mL) 4.80 2.58 6.74 3.93 0.21
IP-10 (pg/mL) 309.30 187.44 278.80 190.82 0.72
MCP-1 (pg/mL) 13.69 5.24 16.29 8.37 1.00
MIP-1 (pg/mL) 29.57 8.91 36.86 26.48 0.43
RANTES (pg/mL) 32.52 24.63 43.24 48.64 0.54
Serum 25(OH)D levels and serum 1,25-dihydroxyvitamin D levels
Changes in circulating 25(OH)D levels in both treatment groups throughout the 4 months follow-up are depicted in Figure 1. From as early as the second day of follow-up women on oral HyD treatment experienced substantially higher serum levels of 25(OH)D levels than those treated with vitamin D3. In the HyD group, the average circulating level of 25(OH)D continued to increase more rapidly than in the vitamin D3 group for the entire follow-up period, reaching by the last clinical visit 69.5ng/mL versus 31.0ng/mL in the vitamin D3 group (mean difference 39.0ng/mL, p<0.0001; Table 2). 25(OH)D levels shifted to above 30ng/mL in all participants of the HyD group at 35 days of follow-up, while in the vitamin D3 group about 50% of participants remained below this threshold throughout the 4 months of follow-up (Fig. 1). Both treatments resulted in an increase in 1,25(OH)2D levels, but this increase was more pronounced with HyD. Average circulating 1,25(OH)2D rose by 19.5pg/mL in the HyD group and by 2.5pg/mL in the vitamin D3 group (p=0.004; Table 2).
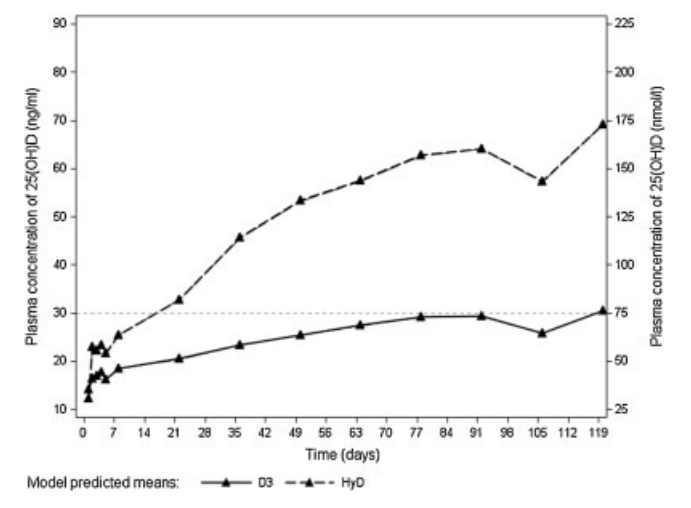
Figure 1.
Change in 25(OH)D over time with HyD versus vitamin D3. Dashed line indicates the 30ng/mL (75nmol/L) threshold of 25(OH)D serum concentration. Graph shows individual and model predicted means of serum 25(OH)D levels across 14 clinical visits by treatment. Predicted means are adjusted for age, BMI, and baseline 25(OH)D levels. 25(OH)D levels shifted to above 30ng/mL in all participants of the HyD group at 35 days of follow-up, while in the vitamin D3 group about 50% of participants remained below this threshold throughout the 4-month follow-up.
Table 2. Treatment Effects on Biomarkers
Repeated measurement analysis using all time points (means (SE) across follow-up) End of follow-up comparison (means (SE) at the end of follow up)
Vitamin D3 HyD Difference Vitamin D3 HyD Difference
All analyses controlled for the results of the same tests at baseline, age, and BMI. All parameters were analyzed by repeated measurement analysis in addition to the end of study comparison. Plasma levels of 25(OH)D and immunity markers were assessed in all 14 visits. Insulin and fasting glucose levels were assessed only at visits 2 (day 2) and 10 (day 63). 1,25-dihydroxyvitamin D levels were assessed at visits 2 (day 2), 9 (day 50), and 14 (day 120).
SE=standard error; HyD=25(OH)D3; PTH=parathyroid hormone; IL=interleukin; IP-10=interferon gamma-induced protein 10kDa; MCP-1=monocyte chemotactic protein-1; MIP-1=macrophage inflammatory protein beta; RANTES=Regulated upon Activation, Normal T-cell Expressed, and Secreted; BMI=body mass index.
25(OH)D level (ng/mL) 22.48 (0.81) 40.85 (0.82) p<0.0001 30.99 (1.59) 69.47 (1.58) p<0.0001
1,25(OH)2D level (pg/mL) 42.44 (1.56) 45.98 (1.47) p=0.15 40.50 (2.91) 53.06 (2.76) p=0.004
Serum calcium (mmol/L) 2.28 (0.02) 2.28 (0.02) p=0.97 2.27 (0.03) 2.27 (0.03) p=0.97
Urinary calcium/creatinine ratio 0.41 (0.03) 0.36 (0.03) p=0.37 0.33 (0.06) 0.33 (0.06) p=0.98
PTH (pg/mL) 53.42 (2.48) 49.94 (2.48) p=0.36 51.68 (3.43) 43.00 (3.43) p=0.09
Insulin (mIU/L) 6.02 (0.48) 6.33 (0.43) p=0.69 6.61 (0.69) 6.21 (0.59) p=0.69
Serum glucose (mmol/L) 5.04 (0.07) 5.19 (0.07) p=0.16 5.03 (0.10) 5.14 (0.10) p=0.40
Plasma eotaxin level (pg/mL) 23.23 (1.23) 20.81 (2.29) p=0.43 30.91 (1.61) 21.07 (1.90) p=0.0002
Plasma IL-8 level (pg/mL) 1.07 (0.05) 1.14 (0.05) p=0.35 1.55 (0.12) 1.35 (0.11) p=0.22
Plasma IL-12 level (pg/mL) 1.23 (0.08) 1.14 (0.08) p=0.49 2.63 (0.32) 0.98 (0.18) p<0.0001
Plasma IP-10 level (pg/mL) 294.40 (37.71) 393.75 (37.90) p=0.10 230.71 (59.76) 279.31 (59.75) p=0.57
Plasma MCP-1 level (pg/mL) 7.11 (0.74) 6.17 (0.75) p=0.40 12.11 (1.19) 7.68 (1.21) p=0.01
Plasma MIP-1 level (pg/mL) 19.85 (0.78) 19.98 (0.79) p=0.91 25.57 (1.79) 19.85 (1.43) p=0.01
Plasma RANTES level (pg/mL) 31.90 (5.32) 38.37 (5.33) p=0.44 26.68 (8.81) 24.47 (8.82) p=0.86
Blood pressure and lower extremity function
Treatment with HyD compared with vitamin D3 lowered systolic blood pressure on average by 5.7mmHg (p=0.002) over 4 months, independent of age, BMI, and baseline systolic blood pressure (Table 3). Systolic blood pressure in the HyD group progressively decreased for the first 3 weeks of follow-up and then stabilized at a lower level for the remainder of follow-up; in the vitamin D3 group, on the other hand, systolic blood pressure remained virtually constant for the entire 4-month follow-up (Fig. 2). Diastolic blood pressure did not differ significantly between the treatment groups and remained constant for the duration of the study (Table 3).
Table 3. Treatment Effect on Blood Pressure
Repeated measurement analysis using all time points (means (SE) across follow-up) End of follow-up comparison (means (SE) at the end of follow up)
Vitamin D3 HyD Difference Vitamin D3 HyD Difference
Treatment with HyD lowered systolic blood pressure on average by 5.7mmHg (p=0.002) compared to vitamin D3 across all time points, adjusted for age, BMI, and baseline systolic blood pressure. Diastolic blood pressure did not differ significantly between the treatment groups.
SE=standard error; HyD=25(OH)D3; BMI=body mass index.
Systolic blood pressure (mmHg) 117.0 (1.0) 111.4 (1.0) p=0.002 119.6 (2.6) 112.4 (2.6) p=0.06
Diastolic blood pressure (mmHg) 69.7 (0.8) 69.5 (0.8) p=0.83 67.8 (1.8) 70.8 (1.8) p=0.24
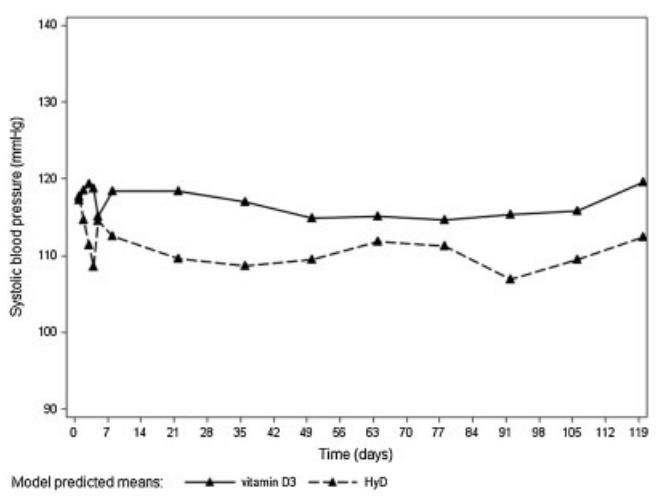
Figure 2.
Change in systolic blood pressure over time with Hyd versus vitamin D3. Graph shows model predicted means of systolic blood pressure across 120 days of follow-up (14 clinical visits) by treatment. Predicted means are adjusted for age, BMI, and baseline systolic blood pressure. Systolic blood pressure decreased in the HyD group progressively for the first 3 weeks of follow-up (up to day 21) and then stabilized at a lower level for the remainder of follow-up; in the vitamin D3 group, on the other hand, systolic blood pressure remained virtually constant for the entire 4-month follow-up.
Among the lower extremity function endpoints, only knee extension strength differed significantly between the treatment groups at 4-month follow-up with a 17% better knee extension strength compared with those treated with vitamin D3 (p=0.03; Table 4), independent of age, BMI, and baseline knee extension strength. Results for the other functional endpoints, including knee flexion strength, timed up-and-go test, and repeated sit-to-stand test, the HyD treatment group yielded somewhat better results (Table 4), but differences between the groups were not statistically significant. Across all tests of lower extremity function, participants in the HyD treatment group had a significantly higher probability to maintain or improve their lower extremity strength/function characteristics (odds ratio [OR]=2.79; 95% confidence interval [CI], 1.18–6.58) during the 4-month follow-up period than those treated with vitamin D3.
Table 4. Treatment Effect on Functional Decline
Characteristic Vitamin D3 group at 4 months (n=10) HyD Group at 4 months (n=10) Difference (%) p
Mean SE Mean SE
At the end of the 4-month follow-up period participants in the HyD group had on average a 17% better knee extension strength compared with those treated with vitamin D3 (p=0.03), independent of age, BMI, and baseline knee extension strength. Results for the other functional characteristics were slightly better in the HyD treatment group, but differences between the groups were not statistically significant. Across all tests of lower extremity function, participants in the HyD treatment group had a significantly higher probability to maintain or improve their lower extremity strength/function characteristics (OR=2.79; 95% CI, 1.18–6.58) during at 4-month follow-up compared with those in the vitamin D3 group.
HyD=25(OH)D3; SE=standard error; BMI=body mass index; OR=odds ratio; CI=confidence interval.
Knee extension strength (N) 247.2 12.1 289.3 12.1 17.01 0.03
Knee flexion strength (N) 180.1 5.7 187.2 5.7 3.93 0.42
Timed up-and-go test (second) 7.9 0.4 7.3 0.4 –7.76 0.32
Repeated sit-to-stand test (second) 8.0 0.5 7.0 0.5 –12.16 0.17
PTH, glucose, insulin, serum calcium, and urinary calcium excretion
Effects of study treatments on circulating concentrations of PTH, glucose, and insulin were not significantly different. However, while glucose and insulin levels stayed stable in both groups for the entire follow-up period, PTH levels decreased in both groups. Figure 3 illustrates that HyD may cause a more rapid decrease in PTH concentration than does vitamin D3. During the 4-month period average PTH levels decreased by 5.7pg/mL in the vitamin D3 group and by 18.1pg/mL in the HyD group; a statistically significant difference was likely missed due to the small sample size.
Figure 3. Change in intact PTH levels over time with Hyd versus vitamin D3. Graph shows model-predicted means of intact PTH levels across five clinical visits by treatment. Predicted means are adjusted for age, BMI, and baseline PTH levels. Based on this figure there is a suggestion that HyD may cause a more rapid decrease in PTH concentration than does vitamin D3. Across the 4-month follow-up, average PTH levels decreased by 5.7pg/mL in the vitamin D3 group and by 18.1pg/mL in the HyD group, but difference between PTH levels in the two treatment groups were not statistically significant throughout the follow-up period. The end of study difference approached significance with a p value of 0.09 (Table 2).
As a safety measure, serum calcium was measured at each study visit. Serum calcium level remained stable throughout the entire 4 months of follow-up and no participant had at any time point a serum calcium level above 2.6nmol/L (reference range: 2.19–2.60nmol/L). Similarly, there was no significant difference in urinary calcium excretion between groups assessed as the calcium/creatinine ratio in spot urine.
Markers of immunity
Treatment effects were significantly different between the treatment groups for four of the seven investigated markers of immunity over the follow up period (Table 2) with a significantly more pronounced suppression of eotaxin, IL-12, MCP-1, and MIP-1 with HyD compared to vitamin D3 at the last visit at 4 months. Notably, however, treatment with either form of vitamin D led to decrease in circulating levels of the markers indicating lowering the level of innate immune activity by both HyD and vitamin D3. In the combined group of participants treated with either form of vitamin D, the overall declining trend had two distinct phases: a relatively precipitous decrease during the first 5 days of follow-up, which was not different between the daily and weekly application, and somewhat slower decline during the 98-day period starting on day 8 (up to day 106); these two periods were separated by the brief rise of circulating levels on days 6 and 7 (Fig. 4).
Figure 4. Change in immunity markers over time with Hyd and vitamin D3 combined. Graph shows model predicted means of all seven tested immunity markers across 14 clinical visits (combined n=20). Predicted means are adjusted for age, BMI, and baseline levels of immunity markers. Serum concentration for IP10 (interferon gamma-induced protein 10kDa) is shown on the right y-axis in the top graph of the panel. The left y-axes show concentrations for all other markers, including eotaxin, interleukin-8 (IL-8), interleukin-12 (IL-12), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein beta (MIP-1), and “Regulated upon Activation, Normal T-cell Expressed, and Secreted” (RANTES). Dashed vertical lines show periods of steady decline. During the first 5 days of follow-up, plasma levels of all investigated immunity markers, except IP-10 and RANTES, decreased; and linear trends estimated by repeated measures mixed models were highly statistically significant (p<0.0001) for all markers except for IP-10 and RANTES. Immunity markers augmented during a 2-day period between day 6 and 8, and after that linear decrease continued. Estimated linear trends were statistically significant (p<0.002) for all markers with the exception of IP-10 for the time interval after day 8 up to day 106.
Discussion
Consistent with previous studies suggesting that HyD is more potent than vitamin D3 in raising 25(OH)D levels,16–21 our study shows that 20µg HyD is significantly more efficient and more rapid in shifting healthy postmenopausal women into a desirable 25(OH)D serum level of at least 30ng/mL compared to 800IU (20µg) vitamin D3. Further, our study adds support to the concept that higher 25(OH)D levels may be more desirable for nonskeletal endpoints of vitamin D, including lower extremity function, blood pressure, and immunity. Although this was a small trial, HyD treatment resulted in a rapid increase in 25(OH)D levels associated with a significant 2.8-fold higher odds of maintained or improved lower extremity function, an immediate and sustained decrease in systolic blood pressure by 5.6mmHg, and a significantly more pronounced suppression of four out of seven markers of innate immunity. Notably, both HyD and vitamin D3 contributed to a decrease in markers of innate immunity, indicating a benefit from both substances.
In this study, participants in the 800IU vitamin D3 group needed about 3 to 4 months to reach a maximum mean concentration of 31ng/mL, leaving about 50% of the participants below the threshold level of 30ng/mL. On the other hand, with HyD at a dose of 20µg, the 25(OH)D threshold of 30ng/mL was achieved in all women already at 35 days of treatment, and after 3 to 4 months, the 25(OH)D level rose to a mean concentration maximum of 69.5ng/mL. As demonstrated by Ilahi and colleagues,27 a rapid response with an early shift to the desired 25(OH)D threshold level of 30ng/mL could also be achieved with vitamin D3; however, only with a much higher oral bolus of 2500µg (100,000IU).
Several lines of evidence support a benefit of vitamin D on blood pressure. First, in experimental studies, 1,25-dihydroxyvitamin D regulates the renin-angiotensin-aldosterone system via suppression of renin gene expression28, 29 and has vasculoprotective effects that inhibits vascular smooth muscle cell proliferation30 and vascular calcification.31 Second, vitamin D3 supplementation (2000IU/d) was found to improve endothelial function in a trial among African American individuals compared to placebo.32 Third, mice lacking the vitamin D receptor (VDR) suffer from hypertension and develop heart hypertrophy.33, 34 Fourth, clinically, in two small trials among patients with mild hypertension and older postmenopausal women, ultraviolet B (UVB) irradiation or vitamin D supplementation (800IU/d) reduced blood pressure by 6 to 9mmHg.35, 36 Notably, a threshold of 25(OH)D that may confer a maximum benefit on blood pressure was suggested in two large cohorts of men and women to be at least 30ng/mL.9 Consistent with these observations, participants treated with HyD (compared to the participants in the vitamin D3 group) reached the 25(OH)D threshold of 30ng/mL within 35 days of treatment and experienced a significant reduction of systolic blood pressure by 5.7mmHg in the same time frame. In fact, the blood pressure reduction occurred in the early treatment phase and stabilized at this level for the remainder of the 4-month follow-up period. Systolic blood pressure did not change in the vitamin D3 group, which may be explained by the much slower and smaller increase in 25(OH)D levels in the vitamin D3 group, leaving 50% of the participants below 30ng/mL even at 4 months of treatment.
Several lines of evidence support a role of vitamin D in muscle health. First, the VDR is expressed in human muscle tissue,37, 38 although this was questioned recently,39 and VDR activation may promote de novo protein synthesis in muscle.40 Mice lacking the VDR show a skeletal muscle phenotype with smaller and variable muscle fibers and persistence of immature muscle gene expression during adult life.34, 41 Second, proximal muscle weakness is a prominent feature of the clinical syndrome of vitamin D deficiency.42 Clinical findings in vitamin D deficiency myopathy include proximal muscle weakness, diffuse muscle pain, and gait impairments such as waddling way of walking.43 Third, vitamin D supplementation increased muscle strength and balance,24, 44 and reduced the risk of falling in community-dwelling participants of double-blind randomized-controlled trials that gave 700 to 1000IU vitamin D per day as summarized in a 2009 meta-analysis.45 Notably, a threshold of 25(OH)D that may confer a maximum benefit on lower extremity function suggested to be at least 24ng/mL and best 30 to 40ng/mL based on the large National Health and Nutrition Examination Survey (NHANES) III study,46 and the same thresholds were suggested for fall prevention based on the 2009 meta-analysis of double-blind randomized controlled trials (RCTs).45
Consistent with the concept of optimal 25(OH)D threshold accomplishment, participants in the HyD group compared to the vitamin D3 group improved in all of four lower extremity tests at 4-month follow-up, although statistical significance was only achieved for knee extensor strength with a 17% improvement. Improvements did not reach statistical significance for knee flexor strength (4%), for functional mobility (timed up-and-go test; 8%) and for reaction time (repeated sit-to-stand test; 12%), which may have been due to the small sample size. Overall, however, participants had a significant 2.8-fold higher odds to maintain or improve their lower extremity function with HyD compared to vitamin D3.
Experimental and observational data support a role of vitamin D in the innate and adaptive immune response.34, 47 1,25-dihydroxyvitamin D treatment of cancer cells suppressed IL-8 production, which is an angiogenesis factor critical in initial tumor and metastasis development.48 Further, 1,25-dihydroxyvitamin D treatment inhibited RANTES and IP-10 secretion in a concentration-dependent manner in airway smooth muscle cells.49 Both chemokines play a critical role in the pathogenesis of asthma,50 as do eosinophils, which are recruited to the lungs by eotaxin.51 Notably, low 25-hydroxyvitamin D levels were associated with elevated total immunoglobulin E (IgE) and eosinophil counts in a larger study of children with asthma.52 Further, in patients with chronic kidney disease–associated inflammation, urinary MCP-1 protein and renal macrophage infiltration were significantly and inversely correlated with 1,25(OH)2D levels.53 MCP-1 is considered a key modulator of monocyte activity and was suggested to be involved in the initiation and progression of atherosclerosis.54 Further, previous studies suggested that 1,25(OH)2D3 stimulates first-line immune defense in the innate immune system, resulting in an increase in maturation of monocyte to macrophage and an increase in MIP-1b.55 Finally, IL-12, a proinflammatory cytokine that plays a central role in activation and differentiation of CD4(+) T cells into interferon- secreting T-helper type 1cells, has been reported to be downregulated by 1,25-dihydroxyvitamin D treatment.55, 56
In our study, all tested markers of innate immunity showed some response to both types of vitamin D, but it was significantly more pronounced with HyD for four markers of innate immunity (eotaxin, IL-12, MCP-1, and MIP-1). The response to both types of vitamin D was very rapid in the first 5 days after treatment initiation, during which all but IP-10 and RANTES decreased, followed by an increase of all markers during a 2-day period between day 6 and 8, and a further decline thereafter. Estimated linear trends were statistically significant for all markers with the exception of IP-10 for the time interval after day 8 up to day 106. Notably, two previous trials that tested vitamin D with treatment doses of up to 40,000IU vitamin D3 per day among patients with multiple sclerosis (MS)57 and obese patients58 were not able to demonstrate any significant change in a number of markers of immunity. The difference in results between these trials and our study may be explained by the follow-up time points documented, which included early response only in our trial.
Our results support the threshold recommendation of 30ng/mL of the IOF and the Endocrine Society as they show that among participants in the HyD group who all reach the threshold within 3 to 5 weeks, greater benefits are observed with regard to lower extremity function, blood pressure, and innate immunity compared with participants of the vitamin D3 group who reach the 30ng/mL threshold only in about 50% of cases after 16 weeks.2, 3
Our trial has several strengths despite its small size. We had complete adherence to the study protocol and 100% of participants attended 14 clinical visits. The trial contributes new data on HyD effects on blood pressure, lower extremity function, and markers of immunity compared to the current standard dose of vitamin D3. The trial was sufficiently powered for the primary endpoint, but had limited power for secondary endpoints, which were included to provide pilot data. However, multiple repeated measurements compensate a small sample size by reducing measurement error to some extent, and allowed for adequate power for some of the secondary endpoints; ie, systolic blood pressure. Notably, as we did not test an equivalent dose of HyD and vitamin D3 with respect to 25(OH)D level increase, benefits documented by HyD may likely be caused by its rapid increase in 25(OH)D level and higher achieved 25(OH)D level compared with the standard dose of vitamin D3 tested. Alternatively, HyD may have additional benefits superior to vitamin D3, which will need further investigation.
In conclusion,
our results show that oral supplementation with HyD (25(OH)D3 metabolite) at a dose of 20µg per day resulted in a safe, immediate, and sustained increase in 25(OH)D levels in all participants. The higher and faster increase in circulating level of 25(OH)D reached with HyD compared with vitamin D3 may explain the benefit of HyD on systolic blood pressure reduction, improvement in lower extremity function, and the more pronounced reduction in several markers of innate immunity among healthy postmenopausal women.
Disclosures
The collaborating authors from DSM Research & Development performed the analysis of 25(OH)D levels blinded to treatment allocation. DSM also performed the biomarker analysis for innate immunity (the authors are grateful to Nathalie Richard), also blinded to treatment. All other measurements and analyses were performed at the Centre on Aging and Mobility at the University of Zurich and the Institute of Clinical Chemistry at the University Hospital in Zurich. DSM had no influence on the design of the study, the content of the publication or the underlying statistical analyses. The principal investigator (Heike Bischoff-Ferrari) was supported exclusively by a Swiss National Foundations Professorship Grant (PP00B-114864). None of the university affiliated co-authors have a conflict to report.
Acknowledgements
This trial was funded by an independent investigator initiated grant provided by DSM Nutritional Products Research & Development and a Swiss National Foundations Professorship Grant (PP00B-114864).
Authors' roles: HBF designed the study with input by BDH, JOE, WCW, HBS, AJ, and ES. Innate immune markers and 25(OH)D were measured at DSM under supervision by ES, SW, and JS; all other biomarkers were measured by AvE. Data collection was performed by AE, JH, and HBF.
See also VitaminDWiki
Application to FDA for use of active Vitamin D for hemodialysis patients – July 2011
Active form of vitamin D appears to help prevent and treat some cancers – Feb 2011
Some form of vitamin D may be the lowest cost treatment for CKD – Spanish Sept 2011
Overview Liver and vitamin D probably should consider Calcidiol if have liver problems or perhaps have had gallbladder removed
Nano-encapsulated of Vitamin D3, Calcidiol, calcitriol look promising, esp time release – Dec 2012 7 day!!
Calcidiol may be 5X more effective than Vitamin D3 – June 2012
Is HyD (25(OH)D) a better form of vitamin D for some animals and maybe humans with liver problems includes DSM recommendations for Vitamin D and HyD
Time-release form of active vitamin D granted a patent for chronic kidney disease – July 2014
Pigs have about 2X less variation in response to calcidiol as humans do to vitamin D – 2015
Calcitriol category listing has items along with related searches
Comment: VitaminDWiki
HyD is a commercial product developed by DSM - years ago for poultry. Some of the HyD product literature is at the bottom of this page.
HyD has a much shorter half-life than vitamin D3 - so should be taken more often (daily instead of weekly or monthly)
Unaware if HyD is available as of Jan 2014 for humans.
