Hypothesis -Tissue barriers, created to protect body, block vitamin D
Vitamin D, Vitamin D Receptor, and Tissue Barriers
Tissue Barriers. ; 1(1): . doi:10.4161/tisb.23118. ~~#009:
Yong-guo Zhang, Shaoping Wu, and Jun Sun jun_sun@rush.edu
Department of Biochemistry, Rush University, 1735 W. Harrison Street, Chicago, IL, 60612, USA
Tissue barriers are critical in the pathogenesis of human diseases, such as
atopic dermatitis,
inflammatory bowel diseases, and
various cancers.
Preserving or restoring barrier functions of the epithelia cells is a therapeutic strategy to prevent and treat the illness. Mounting evidence indicates that vitamin D and the vitamin D receptor (VDR) play key roles in the pathogenesis of human diseases. In particular, we note an interesting link between vitamin D/VDR signaling and tissue barriers. In the current review, we summarize the recent progress on vitamin D and cell junction complexes. We focus on the functions of VDR and VDR-associated intracellular junction proteins, such as P-catenin and claudins. We also discuss the potential therapeutic functions of vitamin D in treating defective tissue barriers that involve skin, intestine, lung, kidney, and other organs. However, the mechanisms for the vitamin D/VDR signaling in tissue barriers remain largely unknown. Further studies on vitamin D/VDR's multiple functions in physiological models will suggest new therapeutic targets for prevention and treatment diseases with defective barrier functions.
Keywords
Adherens junction; P-catenin; cancer; Claudin; E-cadherin; inflammation; tight junction; vitamin D; Vitamin D receptor; ZO-1
The following is most of the text from the PDF. Some of the tables would take too long to extract
PDF is attached at the bottom of this page
Introduction
Classically, vitamin D is known as a key player in calcium homeostasis, electrolyte and blood pressure regulation, and immune response.1 Vitamin D receptor (VDR) is a nuclear receptor2 that mediates most known functions of 1,25-dihydroxyvitamin D (1,25(OH)2D3), the active metabolite of vitamin D. Approximately 3% of the mouse and human genomes are regulated directly or indirectly by the vitamin D endocrine system, suggesting widespread functions of vitamin D and VDR in diseases.3-5 Sunlight exposure is the primary source of vitamin D. Pro-vitamin D is formed in the skin through the action of ultraviolet irradiation. It is then metabolized into two different substances within the body: 25(OH)D, or calcidiol, and 1,25(OH)2D3, or calcitriol. Vitamin D can also be taken from the diet. Decreased sun exposure limits vitamin D synthesis. Current research has implicated that vitamin D deficiency is a critical factor in the pathology of varieties of cancer as well as inflammatory bowel diseases (IBD), bacterial infection, autoimmune diseases, diabetes, osteoarthritis, periodontal disease, skin disorders, and more.6-14
Tissue barriers play an essential role in the pathogenesis of some human diseases. Preserving or restoring barrier functions of the epithelial cells is a therapeutic strategy to prevent and treat the illness. In particular, we note a link between vitamin D/VDR signaling and barrier functions. However, how the vitamin D/VDR signaling is involved in tissue barrier related to human diseases remains largely unknown. In the current review, we will summarize the recent progress in the roles of vitamin D/VDR signaling in diseases associated with vitamin D deficiency and tissue barrier defects. We will discuss the potential therapeutic functions of vitamin D/VDR in the related diseases.
Vitamin D and adherens junctions
Cell adhesion is the binding of a cell to a surface, extracellular matrix or another cell using cell adhesion proteins. Adherens junctions (AJs) represent a multiprotein complex located at the lateral plasma membrane of contacting epithelial cells. Epithelial AJs are composed by transmembrane proteins such as E-cadherin and nectin and cytosolic scaffolds, a-catenin, P-catenin, and p120-catenin. Cellular adhesions are actively involved in signal transduction. P-catenin pathway controls a wide variety of normal and pathological processes, including embryogenesis, differentiation, and carcinogenesis.15-18 Interestingly, the activity of P-catenin can be repressed by activation of VDR.18 The interaction exists between the activator function-2 domain of the VDR and C terminus of P-catenin. Acetylation of the P-catenin C terminus differentially regulates its ability to activate T cell factor (TCF)- or VDR-regulated promoters in vitro.18 Vitamin D3 and VDR affect the Wnt signaling through direct interaction with P-catenin, thus attenuating growth in colon cancer cells.19-21
Cancer cells are characterized by decreased expression of E-cadherin and nuclear relocalization of P-catenin. 1,25(OH)2D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling.22 These were observed in human colon carcinoma SW480 cells expressing VDRs (SW480-ADH) but not in that of a malignant subline (SW480-R) or metastasic derivative (SW620) cells lacking VDR. Taken together, the published data provide the rationle for using vitamin D in anti-cancer . It also indicates the unique role of VDR in regulating structure and signaling functions of adhesion complexes.
Vitamin D and tight junctions (TJs)
The TJs seal the space between adjacent epithelial cells.
TJ structure plays a critical role in
tissue barriers,
host defense, and
inflammation.
TJ protein complexes are composed of integral membrane proteins, cytoplasmic plaque proteins, and cytoskeletal proteins. Among these, the claudin family membrane proteins are key components for the structure and function of TJs.23 Molecular composition of TJs also involves occludin and members of 'zonula occludens' (ZO) protein family, such as ZO-1, ZO-2 and ZO-3. Vitamin D3 induces the expression of occludin, ZO-1, ZO-2, and vinculin. Vitamin D3 treatment promotes the translocation of ZO-1 to the plasma membrane.22 Treatment with 1,25(OH)2D3 is able to abrogate podocytes injury, detected as desmin expression and loss of nephrin and ZO-1. 1,25(OH)2D3 administered with a therapeutic regimen may revert proteinuria, counteracting glomerular podocyte injury.24
Corneal epithelial cells had increased transepithelial resistance (TER), decreased inulin permeability, and increased occludin levels when cultured with 25(OH)D3 and 1,25(OH)2D3.25 Although 1,25(OH)2D3 treatment alone decreased TER in the cultured intestinal epithelial cells,26 1,25(OH)2D3 pretreatment protects cells from increasing permeability induced by Dextran sulfate sodium (DSS) in vitro?2 TJ proteins, such as ZO-1, are upregulated in enterocytes by 1,25(OH)2D3. In the DSS-induced colitis mouse model, VDR- - mice are more susceptible to the DSS treatment compared to the VDR+/+ mice.27 These data indicate that VDR may play a critical role in mucosal barrier homeostasis by preserving the integrity of junction complexes and the healing capacity of the colonic epithelium.27
In vitro, VDR deletion in intestinal epithelial cells leads to decreased claudin 2 and 12 that contributed to vitamin D-dependent calcium homeostasis.26 Claudin-2 is known as a 'leaky' claudin that forms a paracellular water channel and thus mediates paracellular water transport in leaky epithelia.28-32 Christakos et al. also reported that 1,25(OH)2D3 downregulates cadherin-17 and upregulates Claudin 2 and 12 in the intestine. Vitamin D mediated intestinal calcium absorption. These data indicate that 1,25(OH)2D3, by regulating these epithelial cell junction proteins, can redirect transepithelial transport of calcium toward the paracellular pathway.33
We summarize the recent reports on vitamin D and cell structural proteins including E-cadherin, P-catenin, ZO-1, claudins, and Occludin in Table 1. Further insights into the mechanisms responsible for VDR and barrier dysfunction are still needed in both in vitro and in vivo systems.
Human diseases associated with vitamin D deficiency and defective tissue barrier
Vitamin D deficiency is caused by inadequate nutritional intake of vitamin D coupled with inadequate sunlight exposure. VDR is expressed in various tissues and mediates functions of 1,25(OH)2D3, the active metabolite of vitamin D. Therefore, it is not surprising that many human diseases associated with vitamin D deficiency also involve altered signaling and functions of VDR. The traditional model of treating cells with vitamin D3 that guided early vitamin studies is now giving way to a model with more complex mechanisms of action . Furthermore, recent studies provide new evidence for the key role of VDR in the pathogenesis of the diseases and defective tissue barriers. Atopic dermatitis (AD) is a multifactorial, heterogenous disease with defects in epidermal barrier integrity. A recent study has demonstrated that VDR signaling is important in keratinocytes that regulate skin barrier and homeostasis.34
The intestinal epithelial cells play barrier, structural, and host defense roles . Defective epithelial barriers have been implicated in IBD and can predict relapse during clinical remission.28, 35-40 Changes of claudin 2 are associated with active IBD.28, 38 Dysfunction of the junctional adhesion molecule, JAM-A, induced expressions of claudin 10 or 15.41 Genes implicated in mucosal barrier function (ECM1, CDH1, HNF4a, and laminin B1) confer risk of ulcerative colitis (UC); furthermore, E-cadherin is the first genetic correlation between colorectal cancer and UC. Interestingly, these TJ and cell adhesion proteins are associated with VDR. Several recent studies suggest that VDR stabilizes TJ structure and modulates intestinal inflammation27 and VDR status affects the development of Salmonella-colitis and DSS-colitis.42, 43 In addition to VDR-dependent regulation of tight junctions increasing evidence demonstrates that VDR possesses anti-inflammatory activity.44 Delineating the mechanisms that regulate intestinal VDR signaling will help us to understand how to modulate VDR signaling in order to restore normal function of this receptor and reduce chronic inflammation.
In lungs, deficiency of vitamin D is associated with accelerated decline in lung function. Chronic respiratory diseases associated with vitamin D deficiency include cystic fibrosis, interstitial lung disease, and chronic obstructive pulmonary disease (COPD).45-48 Deficiency of vitamin D has also been associated with increased risk of respiratory infection from influenza A and Mycobacterium tuberculosis.49 Absence of VDR in mouse lungs can lead to an early onset of emphysema/COPD because of chronic inflammation, immune dysregulation, and lung destruction.50
However, to our knowledge, there is no report on VDR regulation of cell junction proteins in lungs.
Therapeutic potential of vitamin D/VDR in diseases associated with tissue barrier
Significant progress has been recently achieved in applying vitamin D3 analogs and VDR modulators to treat IBD, skin disorders, chronic kidney disease and other diseases. VDR activators, such as calcitriol, doxercalciferol, paricalcitol, or alfacalcidol, are used to activate the vitamin D signaling pathways. Laverny et al. demonstrated that intrarectal administration of 1,25(OH)2-16-ene-20-cyclopropylvitamin D had beneficial effects in the dextran sodium sulfate (DSS) mouse model, without causing hypercalcemia.51 To reduce the risk of hypercalcemia, Goff et al. used beta-glucuronides of vitamin D to deliver 1,25(OH)2D3 to the colon to ameliorate colitis, while plasma calcium concentrations were lower in mice treated with P-gluc-1,25(OH)2D than in mice treated with 1,25(OH)2D3.52,53 Recently, Miheller et al. reported that two 0.25 ng doses of 1,25(OH)2D3 per day did not cause hypercalcemia and did improve the Crohn's disease activity index in Crohn's patients 6 weeks after treatment.53
Oral vitamin D metabolites have been used to correct hypocalcemia in the chronic kidney disease patients with secondary hyperparathyroidism for many years.54 VDR activators supplementation is used to enhance the VDR activation and is associated with better survival in the chronic kidney disease patients.55, 56
Vitamin D3 analogs have been used in the topical treatment of psoriasis for a long time.57 Cathelicidin antimicrobial peptide is directly regulated by the vitamin D/VDR signaling.58 Vitamin D3 also acts together with parathyroid hormone (PTH), or the shared amino-terminal domain of PTH-related peptide (PTHrP), to synergistically increase cathelicidin and immune defense.59 PTH/PTHrP serves to compensate for inadequate vitamin D during activation of antimicrobial peptides.59 Hata et al. showed increased cathelicidin in skin biopsies in atopic dermatitis patients after oral vitamin D supplementation.60 Calcipotriol or calcipotriene is a synthetic derivative of calcitriol. Calcipotriol treatment could enhance cathelicidin during human skin wounding, which would improve wound healing by increasing re-epithelialization and granulation tissue formation.61 Calcipotriol also increased cathelicidin in lesional skin in psoriasis.62 The healing of wound involves different steps: hemostasis, inflammation, granulation tissue formation, and re-epithelialization. Vitamin D-induced cathelicidin promotes the wound healing. However, we did not find any reports on the direct role of cathelicidin in enhancing tissue barriers.
1,25(OH)2D3 pretreatment enhances the efficacy of allergen immunotherapy in mouse allergic asthma model induced by ovalbumin and aluminium hydroxide.63 Administration of four doses of 2.5 mg vitamin D3 did not affect time to sputum culture conversion in the whole study population of patients with pulmonary tuberculosis, but it did significantly hasten sputum culture conversion in patients with the tt genotype of the TaqI vitamin D receptor polymorphism.64
There is an increasing interest in using vitamin D as an inexpensive and easy supplement for disease prevention. If we do not understand the vitamin D metabolism and VDR mechanisms, vitamin D taken by people may not be used effectively and efficiently. Recently, an alternative vitamin D signaling was reported.65 A vitamin D hydroxyanalogs 20(OH)D(2) has antiproliferative and prodifferentiation activities through activation of VDR in a cell-type dependent manner without detectable toxic calcemic activity.66, 6765 This pathway of vitamin D3 metabolism initiated by CYP11A1(25-OHase) and modified by CYP27B1 (1a-OHase) activity.65 With the increasing insights into the vitamin D signaling pathways in defective tissue barriers, new therapeutic strategies may be developed in the near future.
Conclusion
Recent studies of vitamin D and its receptor revealed different cellular functions of VDR that are based on multiple intracellular signaling pathways and molecular targets of this protein. Specifically, VDR appears to regulate molecular composition and functions of different epithelial junctions. As summarized in Figure 1, VDR has physical interaction with P-catenin. Activation of VDR suppresses the activity of P-catenin, thus deceasing nuclear P-catenin and inhibiting cell proliferation. VDR status is also directly associated with the expression level and functions of TJ proteins, such as claudin2 and 12. Increased VDR level leads to increased claudin2 and 12, which may play roles in calcium homeostasis and barrier function (Figure 1). The other cell junction proteins involved in the vitamin D/VDR include E-cadherin, Occludin, and ZO-1.
Taken together, vitamin D/VDR signaling regulates not only structural integrity but also transport functions of different epithelial barriers. Since preserving or restoring barrier functions of epithelial barrier has clear beneficial effects during tissue inflammation and other diseases, better understanding effects of VDR on epithelial junctions will lead to development of novel therapeutic strategies for human diseases accompanied by disruption of tissue barrier.
Figure 1
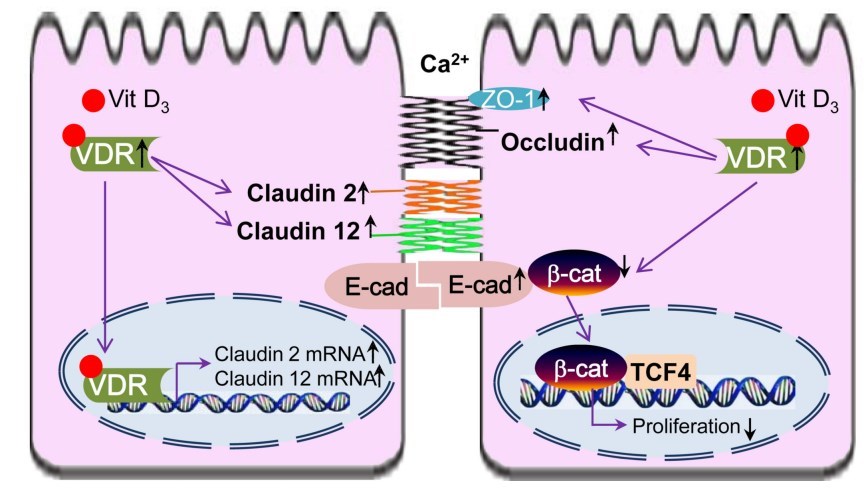
VDR and cell adhesion/TJs of epithelial cells. Activation of VDR suppresses the activity of
β-catenin, thus deceasing nuclear β-catenin, suppressing transcriptional factor T cell factor
(TCF), and inhibiting cell proliferation. Increased VDR level is also associated with
increased claudin2 and 12, which may play roles in calcium homeostasis and barrier
function. The other cell junction proteins involved in the vitamin D/VDR include E cadherin, Occludin, and ZO-1.
Acknowledgments
This work was supported by the NIDDK (KO1 DK075386 and 1R03DK089010-01), the American Cancer Society (RSG-09-075-01-MBC), and the Swim Across America Cancer Research Award to Jun Sun.
References
1. Demay MB. Mechanism of vitamin D receptor action. Ann N Y Acad Sci. 2006; 1068:204-213. [PubMed: 16831920]
2. Haussler MR, Whitfield GK, Haussler CA, Hsieh J-C, Thompson PD, Selznick SH, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone and Mineral Research. 1998; 13:325-349.
3. Wang Y, Becklund BR, DeLuca HF. Identification of a highly specific and versatile vitamin D receptor antibody. Arch Biochem Biophys. 494:166-177. [PubMed: 19951695]
4. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008; 29:726-776. [PubMed: 18694980]
5. Carlberg C, Seuter S. A genomic perspective on vitamin D signaling. Anticancer Res. 2009; 29:3485-3493. [PubMed: 19667142]
6. Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2011; 13:3-19. [PubMed: 21845365]
7. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007; 7:684-700. [PubMed: 17721433]
8. Blaney GP, Albert PJ, Proal AD. Vitamin D metabolites as clinical markers in autoimmune and chronic disease. Ann N Y Acad Sci. 2009; 1173:384-390. [PubMed: 19758177]
9. Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008; 4:404-412. [PubMed: 18594491]
10. Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003; 95:1765-1771. [PubMed: 14652238]
11. Gocek E, Studzinski GP. Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci. 2009; 46:190-209. [PubMed: 19650715]
12. Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008; 3:1535-1541. [PubMed: 18525006]
13. Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008; 9:107-118. [PubMed: 18076342]
14. Campbell FC, Xu H, El-Tanani M, Crowe P, Bingham V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem Pharmacol. 2010; 79:1-9. [PubMed: 19737544]
15. Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007; 13:4042-4045. [PubMed: 17634527]
16. Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010; 138:2101-2114. e5. [PubMed: 20420949]
17. Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997; 275:1787-1790. [PubMed: 9065402]
18. Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006; 21:799-809. [PubMed: 16543149]
19. Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the "Fountain of Youth" to mediate healthful aging. J Steroid Biochem Mol Biol. 2010; 121:88-97. [PubMed: 20227497]
20. Pendas-Franco N, Aguilera O, Pereira F, Gonzalez-Sancho JM, Munoz A. Vitamin D and Wnt/ beta-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res. 2008; 28:2613-2623. [PubMed: 19035286]
21. Larriba MJ, Valle N, Palmer HG, Ordonez-Moran P, Alvarez-Diaz S, Becker KF, et al. The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr Relat Cancer. 2007; 14:141-151. [PubMed: 17395983]
22. Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001; 154:369-387. [PubMed: 11470825]
23. Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010; 2 a002907.
24. Migliori M, Giovannini L, Panichi V, Filippi C, Taccola D, Origlia N, et al. Treatment with 1,25-dihydroxyvitamin D3 preserves glomerular slit diaphragm-associated protein expression in experimental glomerulonephritis. Int J Immunopathol Pharmacol. 2005; 18:779-790. [PubMed: 16388728]
25. Yin Z, Pintea V, Lin Y, Hammock BD, Watsky MA. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011; 52:7359-7364. [PubMed: 21715350]
26. Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, et al. Tight junction proteins claudin-2 and-12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008; 19:1912-1921. [PubMed: 18287530]
27. Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008; 294:G208-G216. [PubMed: 17962355]
28. Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007; 56:61-72. [PubMed: 16822808]
29. Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010; 123:19131921. [PubMed: 20460438]
30. Schulzke JD, Fromm M. Tight junctions: molecular structure meets function. Ann N Y Acad Sci. 2009; 1165:1-6. [PubMed: 19538280]
31. Van Itallie CM, Holmes J, Bridges A, Anderson JM. Claudin-2-dependent changes in noncharged solute flux are mediated by the extracellular domains and require attachment to the PDZ-scaffold. Ann N Y Acad Sci. 2009; 1165:82-87. [PubMed: 19538292]
32. Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006; 6:581-588. [PubMed: 16458081]
33. Christakos S, Dhawan P, Ajibade D, Benn BS, Feng J, Joshi SS. Mechanisms involved in vitamin D mediated intestinal calcium absorption and in non-classical actions of vitamin D. J Steroid Biochem Mol Biol. 2010; 121:183-187. [PubMed: 20214989]
34. Hartmann B, Riedel R, Jorss K, Loddenkemper C, Steinmeyer A, Zugel U, et al. Vitamin D receptor activation improves allergen-triggered eczema in mice. J Invest Dermatol. 2012; 132:330-336. [PubMed: 21938012]
35. Hollander D. Crohn's disease--a permeability disorder of the tight junction? Gut. 1988; 29:16211624. [PubMed: 3065154]
36. Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, et al. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 2009; 1165:294-300. [PubMed: 19538319]
37. Ivanov AI, Nusrat A, Parkos CA. The epithelium in inflammatory bowel disease: potential role of endocytosis of junctional proteins in barrier disruption. Novartis Found Symp. 2004; 263:115-124. discussion 24-32, 211-8. [PubMed: 15669638]
38. Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008; 88:1110-1120. [PubMed: 18711353]
39. Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000; 35:1163-1169. [PubMed: 11145287]
40. Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993; 341:1437-1439. [PubMed: 8099141]
41. Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007; 204:3067-3076. [PubMed: 18039951]
42. Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, et al. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012; 12:57. [PubMed: 22647055]
43. Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010; 177:686-697. [PubMed: 20566739]
44. Sun J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol. 2010; 26:591-595. [PubMed: 20639756]
45. Agrawal T, Gupta GK, Agrawal DK. Vitamin D deficiency decreases the expression of VDR and prohibitin in the lungs of mice with allergic airway inflammation. Exp Mol Pathol. 2012; 93:7481. [PubMed: 22537547]
46. Finklea JD, Grossmann RE, Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr. 2011; 2:244-253. [PubMed: 22332056]
47. Mak G, Hanania NA. Vitamin D and asthma. Curr Opin Pulm Med. 2011; 17:1-5. [PubMed:21045696]
48. Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011; 183:13361343. [PubMed: 21297070]
49. Finklea JD, Grossmann RE, Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr. 2012; 2:244-253. [PubMed: 22332056]
50. Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011; 406:127-133. [PubMed: 21300024]
51. Laverny G, Penna G, Vetrano S, Correale C, Nebuloni M, Danese S, et al. Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunol Lett. 2010; 131:49-58. [PubMed: 20350569]
52. Goff JP, Koszewski NJ, Haynes JS, Horst RL. Targeted delivery of vitamin D to the colon using beta-glucuronides of vitamin D: therapeutic effects in a murine model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2012; 302:G460-G469. [PubMed: 22114117]
53. Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis. 2009; 15:1656-1662. [PubMed: 19408329]
54. Valdivielso JM, Cannata-Andia J, Coll B, Fernandez E. A new role for vitamin D receptor activation in chronic kidney disease. Am J Physiol Renal Physiol. 2009; 297:F1502-F1509. [PubMed: 19625376]
55. Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008; 74:1070-1078. [PubMed: 18633342]
56. Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005; 16:1115-1125. [PubMed: 15728786]
57. Dombrowski Y, Peric M, Koglin S, Ruzicka T, Schauber J. Control of cutaneous antimicrobial peptides by vitamin D3. Arch Dermatol Res. 2010; 302:401-408. [PubMed: 20221619]
58. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005; 19:1067-1077. [PubMed: 15985530]
59. Muehleisen B, Bikle DD, Aguilera C, Burton DW, Sen GL, Deftos LJ, et al. PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection. Sci Transl Med. 2012; 4:135ra66.
60. Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008; 122:829-831. [PubMed: 19014773]
61. Heilborn JD, Weber G, Gronberg A, Dieterich C, Stahle M. Topical treatment with the vitamin D analogue calcipotriol enhances the upregulation of the antimicrobial protein hCAP18/LL-37 during wounding in human skin in vivo. Exp Dermatol. 2010; 19:332-338. [PubMed: 19878298]
62. Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Buchau A, et al. Vitamin D analogs differentially control antimicrobial peptide/"alarmin" expression in psoriasis. PLoS One. 2009; 4:e6340. [PubMed: 19623255]
63. Ma JX, Xia JB, Cheng XM, Wang CZ. 1,25-dihydroxyvitamin D(3) pretreatment enhances the efficacy of allergen immunotherapy in a mouse allergic asthma model. Chin Med J (Engl). 2010; 123:3591-3596. [PubMed: 22166637]
64. Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011; 377:242-250. [PubMed: 21215445]
65. Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2010; 300:C526-C541. [PubMed: 21160030]
66. Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012; 44:2003-2018. [PubMed: 22877869]
67. Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012; 26:3901-3915. [PubMed: 22683847]
68. Ordonez-Moran P, Alvarez-Diaz S, Valle N, Larriba MJ, Bonilla F, Munoz A. The effects of 1,25-dihydroxyvitamin D3 on colon cancer cells depend on RhoA-ROCK-p38MAPK-MSK signaling. J Steroid Biochem Mol Biol. 2010; 121:355-361. [PubMed: 20223287]
69. Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular Mechanisms of Vitamin D Action. Calcif Tissue Int. 2012
70. Pereira F, Barbachano A, Silva J, Bonilla F, Campbell MJ, Munoz A, et al. KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells. Hum Mol Genet. 2011; 20:4655-4665. [PubMed: 21890490]
See also VitaminDWiki
almost nothing - this is a new concept
Wonder if this accounts for the approximate 4X more vitamin D needed to treat a disease (which has a barrier)
