Hepatitis B virus might be treated by Vitamin D
The Role of Vitamin D in HBV infection
European Journal of Biotechnology and Bioscience 2015; 3 (2): 35-41
Saeeda Baig, Shamim Mushtaq, Syed Zaryab Ahmed, Moazzam Ali Shahid
Hepatitis B virus (HBV) infection, 30 years later since the launch of its vaccine, is still a global health Problem. The infection by HBV is a multiplex process dependent on host immune response involving the genetic milieu of the host for proliferation. In acute self-limiting hepatitis, a broad T-cell immune response occurs that is strong enough to eradicate the virus or suppress viral replication. The role of T-cell in the immunopathogenesis of HBV is to control HBV replication, destroy hepatocytes infected by HBV and ultimately eradicate infection. Vitamin D (1, 25(OH) D3) with its autocrine, intracrine and endocrine functions, is an important regulator of immune function, affecting both innate and adaptive immunity. T cells are richly provided with vitamin D receptor (VDR) and have been shown to play a critical role in the pathogenesis of viruses. The toll like receptors (TLR-2) activated by HBV attack, stimulates CYP27B1 (1a-hydroxylase) enzyme within macrophages to convert vitamin 25(OH)D2 to 1,25(OH)D3 which increases regulatory T cells and enhances the secretion of IL-10, and decreases the IL-2 release from dendritic cells. In addition it transcribes mRNAs for the synthesis of antimicrobial proteins which help in the phagocytosis of the HBV pathogens. This systematic review of the literature focuses on the role of vitamin D on the course of HBV infection. How vitamin D regulates the development and function of the immune complexes and the phagocytic proteins to control the clearing of the HBV infection is assessed and discussed.
📄 Download the PDF from VitaminDWiki
Figure 2
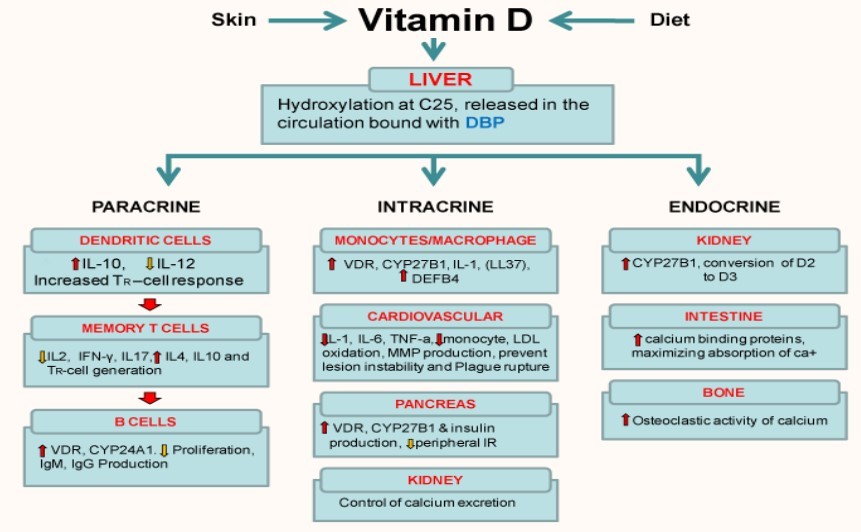
Introduction
Hepatitis B virus (HBV) infection, 30 years later since the launch of its vaccine, is still a global health Problem. The outcome of HBV infection depends on host-pathogen interactions involving the genetic milieu of the host and his immune system [1]. HBV is a common infectious disease of the liver, remains the major cause for complex hepatic diseases, and is endemic particularly in developing countries, affecting 240 million people worldwide with an estimated 600,000 deaths per year [2]. Acute hepatitis of varying severity exists in 95% of children and 2-10 % of adult population [3]. Chronic HBV infection is currently the most common cause of cirrhosis and hepatocellular carcinoma (HCC) in the world. Several mutations and core promoter changes result in 15 to 40% of chronically infected people acquiring serious complications, whereas, the remaining individuals become asymptomatic carriers [4]. The individual difference in clinical presentations of HBV infection in various people is due to differences in host immune response of the person. The host immune system, in particular, the cellular immune response involving immunomodulatory metabolites such as Cytokines and regulatory molecules, play a fundamental role in the immunopathogenesis of HBV infection [5]. There are several hypotheses but exact cellular mechanism(s) is not yet completely understood [6]. Among these metabolites Vitamin D3, 1, 25-dihydroxycholecalceferol (1, 25(OH) D3) is proven to provide prompt immune response. Hepatocytes are richly provided with 1,25(OH)D3 receptors (VDR) and so do sinusoidal endothelial cells, Kupffer cells, stellate cells of normal liver, and the biliary cell line which suggest its role in hepatocellular injury [7]. The immunomodulatory action of 1,25(OH)D3 activates monocytes, increases cell-regulated immunity, inhibits lymphocyte proliferation, immunoglobulin, and cytokine synthesis, and inhibits type 1 cytokine secreting T helper (Th1) response while activating Th2 response [8]. This systematic review of the literature focuses the current understanding of role of immunity on the course of HBV infection and how vitamin D regulates the development and function of the immune complexes and the phagocytic proteins to control the clearing of the HBV infection is assessed and discussed.
Discussion
Pathogenesis of HBV
Following the entry of HBV, the body responds with a coordinated innate and adaptive reaction, activating humoral and cellular immune systems. The clinical route of HBV is determined by the interaction between viral replication and the host immune response. HBV enters hepatocytes and uses transcriptional machinery of the hepatocyte to synthesize mRNAs for its DNA and envelop proteins. HBsAg particles and virions produced by reverse transcription in hepatocytes are presented on surface of the cells directly in association with class I MHC. These infected cells are lysed and taken up by macrophages that present these peptides in association with class I and class II MHC molecules. Dendritic cells (DCs) which are potent antigen-presenting cells (APC) and are key modulators of the immune response process the viral peptides from infected cells and present these in association with class I and II MHC molecules. DCs present signals, which are integrated by the T cells and determine the final outcome of T-cell activation. The HBV antigens are processed by APCs and presented to naive T cells by major histocompatibility complex (MHC) molecules which deliver a primary signal to initiate T-cell activation by engaging the T-cell receptor/CD3 complex with foreign antigens associated with MHC molecules. CD8-T cells recognize the infected cell which presents viral proteins, release cytokines TNF-a and IFN-y to inhibit viral replication. CD4-T cells bind with MHC class II molecule and activate CD8-T cell which bind with MHC class I molecule and release IFN-y and TNF-a. [9 10] Figure 1.
Figure 1- 1 .HBV encounter and enters hepatocytes.2.It uses DNA of hepatocyte to replicate its DNA and forms RNA (HBV) cores (HBV peptides). 3.a) These peptides are presented on surface of the cells directly in association with class I MHC. b) These infected cells are lysed and taken up by macrophages who present these peptides in association with class I and class II MHC molecules. 4.C8-T cells recognize the infected cell which presents viral proteins, release cytokines IFN-y and TNF-a.TNF alfa and INF gama that inhibit viral replication. 5.APC macrophage processes the viral peptides from infected cells that it has taken up and present there in association with class I and II MHC molecules. 6.CD 4 T cells bind with MHC class II molecule and activate CD 8 +T cell. 7.CD8 +T cell bind with NHC class I molecule and release INF- gama and TNF alfa.
Role of Toll like Receptors (TLRs)
The transmembrane pattern recognition receptors (PRR), such as Toll like receptors (TLR) are expressed on immune cells (monocytes, macrophages and polymorphonuclear cells). These receptors interact with viral nucleic acids to activate an inflammatory (IL-6, IL-1b & TNF) or the immune responses (both humoral & cellular) to HBV antigens that could lead to viral clearance during infection and HBV pathogenesis. Among the TLRs, TLR3 recognizes viral double-stranded RNA, TLR9 detects viral DNA while TLR7 and TLR8 identify viral single-stranded RNAs [11] Major-histocompatibility-complex (MHC) class II-restricted, CD4+ helper T cells contribute to generation of antibodies against viral envelope antigens those clear circulating virus particles. MHC class I-restricted, CD8+ cytotoxic T lymphocytes eliminate infected cells antimicrobial response in the host [12].
Progression of HBV
Hepatitis B infection is based on the host immune response. The resulting acute hepatitis is self-limiting in individuals with strong immunity leading to protective memory B cells [5]. It is only 1-5% of individuals who develop chronic infection following acute HBV infection. In approximately 10% of new infections, basically in immunocompromised individuals, HBsAg persists in the serum for greater than six months and chronic HBV infection is established [13]. Some 5% of immunocompetent individuals may develop necro- inflamatory liver disease which later on might lead to Cirrhosis and HCC. Thus, HBV is a noncytopathic virus, which evades the cellular immunity to cause persistent infection [5]. A number of other factors, including genetics of the host, or environmental causes and insults such as hygiene, nutrition, treatment, vaccination and pathogen- related features like viral load, genotypes, geographic location may affect the outcome of HBV infection [14]. Reviewing host related immune factors reveal that cytokines and regulatory molecules play a fundamental role in the immunopathogenesis of HBV infection.
Immunomodulatory Response to HBV
The major role is played by T-cells which not only destroy hepatocytes infected by HBV, but also control HBV replication or eradication. In acute hepatitis the T-cells response to HBV is dynamic, polyclonal, and multispecific, ultimately leading to the clearance of the virus. On the other hand weak or poor T-cells response aggravate the HBV infection and may lead to complex liver disorders [15]. Large genome wide association studies have confirmed that genetic variants in the genes involved in immune response are strong predictors of outcome of viral Hepatitis.
Cytokines
Cytokines, the major group of immune molecules, the major player in the initiation and regulation of immune responses, might affect susceptibility of HBV’s natural course of the infection. Though, cytokines include a large number however, polymorphisms of specific genes or receptor of IL- 1, IL-8, IL-10, IL-18, IL-28B, tumor necrosis factor-a (IFN- a), interferon-y (IFN-y), tumor growth factor-p1, or vitamin D receptor and chemokine receptor affect the clinical course of chronic hepatitis B virus (HBV) infection [15]. Dendritic cells and HBV infected cells are the main source of IFN-a/p, whereas, type 1 IFNs are triggered directly by the presence of viral RNA or DNA synthesized during viral replication. Frequencies of gamma interferon (IFN-y) positive CD8+T cells in inactive HBV surface antigen in HBsAg carriers show a much stronger core-protein-specific cytotoxic T cell response than other types of chronically infected patients [10].
The T memory lymphocytes are imperative in the immune pathogenesis of chronic HBV infection. Thus activation of innate components of immune system seems to represent a key element in control of the initial HBV burst [17].
The proinflammatory cytokine, IL-1 defend against HBV infection by increasing the production of IL-1 p which increases the production of other cytokines; IL-2, IL-6, and TNF-a and stimulate the clearance of HBV. However, subjects with IL-ip (-511) polymorphic genotype CC have a high risk of cirrhosis and end stage liver disease or HCC because this genotype has been closely related with HBV- DNA replication [18] and is a genetic indicator of hepatocellular cancer development in chronic HBV-infected patients [19]. IL-6 is a cytokine involved in cellular proliferation and differentiation. It is produced by a variety of cells such as macrophages, B and T cells and fibroblasts, and thus plays a central role in the inflammatory response associated with the course of chronic hepatitis due to HBV [20]. Similarly, other cytokines and their polymorphic genotypes such as IL-6 [21]), IL-8 [22]), IL-10 [23]), IL-18 [24], IL-28B [25] have been found to influence HBV infection.
Vitamin D combating infection
Vitamin D (1, 25(OH) 2D) with its autocrine, intracrine and endocrine functions, is an important regulator of immune function, affecting both innate and adaptive immunity (Figure 2). In healthy individuals, vitamin D is synthesized in the skin or acquired via the diet. Active D3 requires to be hydroxylated at two positions, C1 and C25. The first C25- hydroxylation of vitamin D occurs in the liver. The final activation by hydroxylation occurs principally in the kidney [26] and also at other sites including the prostate, breast, colon, macrophages etc [27, 28].
Although vitamin D has been studied for over 100 years (1922, Edward Mellanby), the link between vitamin D and immunity has become known for only three decades. Until 1980, vitamin D was recognized as a vitamin to promote calcium homeostasis and bone Health through its classical actions of enhancing calcium absorption in the small intestine, resorption from bone and controlling excretion in the kidney. In 1983, vitamin D3 ( 1,25-(OH)2D3) receptors were discovered on activated Human peripheral blood monocytes (T cells) [29] and established lines of malignant B, T, and non-B, non-T human lymphocytes, as well as in T and B lymphocytes obtained from normal humans and activated in vitro [30].
Mechanism of action of vitamin D
Vitamin D is activated by stimulation of TLRs. They are found on many cells including macrophages, dendritic cells and epithelial cells. In humans, TLR2/1 and TLR4 when triggered result in the induction of the CYP27B1 (1a- hydroxylase) enzyme. This converts 25OH-vitamin D to active vitamin D3 (1, 25(OH) D). The 1,25(OH)2D binds to the vitamin D receptor along with retinoid X receptors (RXR) which then bind to vitamin D-response elements (VDRE) unlocking the DNA, targeting genes transcribing mRNA that encode proteins like cytokines (ILs) Cathelicidin, defensins etc. Cathelicidin is positively charged, it disrupts the viral envelop in a way similar to the breakdown of bacteria in macrophages. Defensin acts as chemoattractants for T cells.TH-1 are inhibited by T regulatory cells (Treg) and protein synthesized (IL-2 and TNFa) from macrophages via VDR activation. Treg increase secretion of IL10 and TGF-P which inhibit TH-1 cell. TH-1 decrease IL-2 secretion inhibiting cellular response. These interlukins also inhibit B-cells producing Immunoglobulins and activates TH-2 cells to increase synthesize of IL4, 5, 10 and 13. This shifts the response from TH-1 to TH-2 cell type. Antibodies synthesized by plasma cells bind to viral antigens and infected hepatocytes giving signals for its destruction [31]. (Figure 3)
Effect of deficiency and supplementation of vitamin D on Hepatitis
Vitamin D deficiency is associated with Acute and chronic HBV and may cause the stimulation of antiviral immune response. Patients with liver cirrhosis are known to be at high risk for vitamin D deficiency in direct proportion to the severity of their chronic liver disease [32]. It has also preventive effect on necroinflammation and liver fibrosis Therefore, it may affect path of HBV infection [33]. Even in conditions that require long-term use of the HBV-active drug, such as in HIV-HBV-coinfection, routine vitamin D assessment and supplementation should be considered necessary because it leads to hypovitaminosis D even in patients who live in the tropics [34]. Vitamin D deficiency may increase viral replication and diminished 25-D3 levels which may portends a poor prognosis in patients infected with HBV [35]. Abu-Mouch and colleagues discovered that by adding vitamin D to conventional Peg-a-2b/ribavirin therapy of chronic HCV genotype 1 patients significantly improves the viral response [36] and this should be a regular practice for all hepatitis patients.
Vitamin D receptor (VDR) Gene Polymorphism in HBV
Gene polymorphisms such as the single nucleotide polymorphism (SNP; replacement of a nucleotide with another one) may change the structure and biological function of the protein coded by that gene. A SNP in the promoter region of a gene may cause increased or decreased production of the relevant protein. The presence of these types of inherited gene polymorphisms may make a person more susceptible or resistant to a certain disease such as chronic viral hepatitis [37]. A member of the nuclear receptor family of transcription factors, VDR expression in hepatocytes suggests its role in hepatocellular injury. Several polymorphisms have been identified in the VDR gene, and their functional significance and potential effects on disease susceptibility have been investigated [58]. The VDR binds the biologically active form of vitamin D, 1, 25- dihydroxyvitamin D or calcitriol and interacts with specific nucleotide sequences of target genes to produce a variety of biologic effects. VDR polymorphisms have been investigated in the context of some chronic liver diseases. Four polymorphisms of the VDR gene have been found associated with various immune diseases. Gene polymorphism of VDR genotype tt is significantly less frequent in patients positive for hepatitis B surface antigen, whereas VDR a/a allele is associated with severity of HBV-related liver disease and with higher viral load. It was suggested that genotype tt provides resistance to chronicity of HBV infection [39, 40]. On the other hand Falleti et al [41] observed that patients affected by liver cirrhosis of viral (HBV or HCV) and alcoholic origin did not demonstrate any differences in the allelic or genotypic frequencies of the VDR polymorphisms compared to controls but differences in the VDR polymorphisms in patients with liver cirrhosis in relationship to the etiology of their liver disease were detected.
Vitamin D deficiency mediated antimicrobial response in HBV
The mammalian Toll-like receptor (TLRs) homologs, including the TLR2 and TLR1 heterodimer [42], recognize a variety of microbial-derived ligands which also includes bacterial lipopeptides. Activation of TLRs results in a direct antimicrobial response in monocytes and macrophages. Various human body cells including lung, colon and prostate cancer cells contain alpha 1 hydroxylase (1a (OH) ase) enzyme that produce 1, 25(OH) D3 which in turn binds nuclear receptor VDR. Activation of 1, 25(OH) D3 results in the production of cathelicidin, an antimicrobial peptide involved in the killing of mycobacteria [43]. Vitamin D deficiency or polymorphism of VDR or CYP27B1 (1a- hydroxylase) enzyme dull the macrophages ability to produce antimicrobial peptides [44]. Liu et al [28]. had previously also shown that TLR activation up-regulated VDR expression and subsequent induction of cathelicidin. Ojaimi et al [45] demonstrated that optimal vitamin D levels after supplementation may improve the expression of TLR2 and hence the body’s ability to fight infections. They have also reported a marked reduction in the induced cytokine profile, specifically IL6, TNF and IFN alpha associated with higher vitamin D levels and the reversibility of the innate immune profile with decreasing levels.
Conclusion
Before the advent of revolutionary pharmaceutics clinicians used to believe in the healing power of sunlight and exposed patients to sun for treatment. Decades later we understand the role of 1, 25(OH) D3 as a sunlight agent that boost up the immune system against a spectrum of diseases that we are gradually recognizing. Today the circulating 1, 25(OH)D3 or vitamin D3 is known to function as a hormone influencing all systems of the body from wound/infections repairs to UTI cure, playing a major role in the prevention of viral, bacterial or fungal infections,.
References
Thursz MR. Host genetic factors influencing the outcome of hepatitis. J Viral Hepat 1997; 4:215-220.
World Health Organization. Hepatitis B. Fact Sheet No 204. July 2013. Available from: http ://www.who.int/mediacentre/factsheets/fs204/en/
Bowyer SM, Sim GM. Relationship within and between the genotypes of Hepatitis B virus at point across the genome: footprints of recombination in certain isolates. J Gen Virol 2000; 81:379-392.
Huang Y, Lok AS. Viral factors and outcomes of chronic HBV infection. Am J Gastroenterol 2011; 106(1):93-95. [DOI: 10.1038/ajg.2010.404]
Rehermann B. Intrahepatic T cells in hepatitis B viral control versus liver cell. Injury J Exp Med 2000; 191(8):1263-1268. [PMCID: PMC2193128]
Sodsai P, Surakiatchanukul T, Kupatawintu P, Tangkitvanich P, Hirankarn N. Association of cytokine and cytokine receptor gene polymorphisms with the risk of chronic hepatitis B. Asian Pac J Allergy Immunol 2013; 31:277-85. DOI10.12932/AP0284.31.4.2013
Luong KV, Nguyen LT. The role of vitamin D in autoimmune hepatitis. J Clin Med Res 2013; 5(6):407- 415.
Tungbilek S. Relationship between cytokine gene polymorphisms and chronic hepatitis B virus infection. World J Gastroenterol 2014; 20(20):6226-6235. [DOI: 10.3748/wj g.v20.i20.6226]
Ganem D, Prince AM. Hepatitis B virus infection - natural history and clinical consequences. N Engl J Med 2004; 350(11):1118-1129.
Cao W, Qiu Z, Zhu T, Li Y, Han Y, Li T. CD8+ T cell responses specific for hepatitis B virus core protein in patients with chronic hepatitis B virus infection. J Clin Virol 2014; 61(1):40-46. [DOI: 10.1016/jjcv.2014.06.022]
Kawai T. Akira S: Antiviral signaling through pattern recognition receptors. J Biochem 2007; 141(2):137-145.
Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 2008; 4:80-90. [DOI: 10.1038/ncpendmet0716]
Hoofnagle JH, Schafer DF. Serologic Markers for Hepatitis B Virus Infection. Seminars in Liver Disease, 1998, 6(1).
Baig S. Molecular Epidemiology of Hepatitis B Virus in Pakistan: Link with Southern Asia. Pak J Med Dent 2013; 2(3):38-42.
Rehermann B. Immunopathogenesis of viral hepatitis. Baillieres Clin Gastroenterol 1996; 10:483-500.
Kim SS, Cheong JY, Lee D, Lee SK, Kim MH, Kwack K et al. Interleukin- 1h and interleukin-1 receptor accessory protein gene polymorphisms are associated with persistent hepatitis B virus infection. Hepatogastroenterology 2012; 59(113): 190-197. [DOI: 10.5754/hge10375]
Katze MG, He Y, Gale M Jr. Viruses and interferon: a fight for supremacy. Nat Rev Immunol 2002; 2(9):675- 687.
Zhang PA, Li Y, Xu P, Wu JM. Polymorphisms of interleukin-1B and interleukin-1 receptor antagonist genes in patients with chronic hepatitis B. World J Gastroenterol 2004; 10(12):1826-1829.
Hirankarn N, Kimkong I, Kummee P, Tangkijvanich P,Poovorawan Y. Interleukin-1beta gene polymorphism associatedwith hepatocellular carcinoma in hepatitis B virus infection. World J Gastroenterol 2006; 12(5):776- 779.
Kao JT, Lai HC, Tsai SM, Lin PC, Chuang PH, Yu CJ et al. Rather than interleukin-27, interleukin-6 expresses positive correlation with liver severity in naive hepatitis B infection patients. Liver Int 2012; 32:928-936. [DOI: 10.1111/j.1478-3231.2011.02742.x]
Giannitrapani L, Soresi M, Balasus D, Licata A, Montalto G. Genetic association of interleukin-6 polymorphism (-174 G/C) with chronic liver diseases and hepatocellular carcinoma. World J Gastroenterol 2013; 19:2449-2455.[PMID: 23674845 DOI: 10.3748/wjg.v19.i16.2449]
Qin X, Deng Y, Liao XC, Mo CJ, Li X, Wu HL. The IL- 8 gene polymorphisms and the risk of the hepatitis B virus/infected patients. DNA Cell Biol 2012; 31:11251130 [PMID: 22335768 DOI: 10.1089/dna.2011.1438]
Zhu QR, Ge YL, Gu SQ, Yu H, Wang JS, Gu XH et al. Relationship between cytokines gene polymorphismand susceptibility to hepatitis B virus intrauterine infection. Chin Med J (Engl) 2005; 118:1604-1609.[PMID: 16232344]
Migita K, Sawakami-Kobayashi K, Maeda Y, Nakao K, Kon-doh S, Sugiura M et al. Interleukin-18 promoterpolymorphisms and the disease progression of Hepatitis B virus-related liver disease. Transl Res 2009; 153:91-96. [PMID: 19138654 DOI: 0.1016/j.trsl.2008.11.008]
Li W, Jiang Y, Jin Q, Shi X, Jin J, Gao Y et al. Expression and gene polymorphisms of interleukinB and hepatitis B virus infection in a Chinese Han population. Liver Int 2011; 31:1118-1126.[PMID: 21745278 DOI: 10.1111/j. 1478-3231.2011.02507.x]
Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys 2007; 460:213-217.[PMID: 17254541 PMCID: PMC2698590]
Reichel H, Koeffler HP, Norman AW. Synthesis in vitro of 1, 25-dihydroxyvitamin D3 and 24, 25- dihydroxyvitamin D3 by interferon-gamma-stimulated normal human bone marrow and alveolar macrophages. J Biol Chem 1987; 262:10931-10937. [PubMed]
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR et al. Toll-like receptor triggering of a vitamin D- mediated human antimicrobial response. Science 2006; 311:1770-1773. [PMID: 16497887]
Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25- dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab 1983; 57(6):1308-1310. [PMID: 5313738]
Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC1. 25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983; 221(4616):1181-1183
Ojaimi S, Skinner NA, Strauss BJG, Sundararajan V, Woolley I, and Visvanathan K. Vitamin d deficiency impacts on expression of toll-like receptor-2 and cytokine profile: a pilot study. J Transl Med. 2013; 11: 176. Published online Jul 22, 2013. doi: 10.1186/14795876-11-176
Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol 2007; 5:513-520. [DOI: 10.1016/j.cgh.2006.10.015]
Motor S, Koksaldi-Motor V, Dokuyucu R, Ustun I, Evirgen O, Yilmaz N et al. Investigation of vitamin D levels in patients with inactive hepatitis B virus carrier. Acta Medica Mediterr 2014, 30:793-796.
Avihingsanon A, Apornpong T, Ramautarsing RA, Ubolyam S, Tangkijvanich P, Ananworanich J. Decline in serum 25 hydroxyvitamin D levels in HIV-HBV- coinfected patients after long-term antiretroviral therapy. Antivir Ther 2014; 19(1):41-9. doi: 10.3851/IMP2673. Epub 2013 Aug 23.
Demir C, Demir M. Vitamin D levels in patients with chronic hepatitis B virus infection and naturally immunized individuals. Internal Medicine Inside 2013, (1)1-4. [DOI: 10.7243/2052-6954-1-2]
Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)- naive patients. World J Gastroenterol 2011; 17:51845190. [DOI: 10.3748/wjg.v17.i47.5184]
De Andrade DR. The influence of the human genome on chronic viral hepatitis outcome. Rev Inst Med Trop Sao Paulo 2004; 46:119-126. [PMID: 15286811]
Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiological of Vitamin D receptor variants. Epidemiol Rev 2000; 22(2):203-217. [PMID: 11218372]
Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, Whittle HC et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis 1999; 179:721-724. [PMID: 9952386]
Suneetha PV, Sarin SK, Goyal A, Kumar GT, Shukla DK, Hissar S. Association between vitamin D receptor, CCR5, TNF-alpha and TNF-beta gene polymorphisms and HBV infection and severity of liver disease. J Hepatol 2006; 44:856-863. [DOI: 16545485]
Falleti E, Bitetto D, Fabris C, Cussigh A, Fontanini E, Fornasiere E, et al. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J Gastroenterol 2010; 16(24):3016-3024. [PMID: 20572305]
Modlin RL, Cheng G. From plankton to pathogen recognition. Nat. Med 2004, 10, 1173.
Reichrath J. Vitamin D, and the skin: an ancient friend, revisited. Exp Dermatol 2007; 16:618-625. [DOI: 10.1111/j. 1600-0625.2007.00570.x]
Holick MF. Vitamin D: Extraskeletal Health. Endocrinol Metab Clin N Am 2010; 39(2):381-400.
Ojaimi S, Skinner NA, Strauss BJ, Sundararajan V, Woolley I, Visvanathan K. Vitamin D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: a pilot study. J Transl Med 2013; 11:176. [DOI: 10.1186/1479-5876-11-176]
