Vitamin D and its active metabolites – DeLuca
History of the discovery of vitamin D and its active metabolites
BoneKEy Reports 3, Article number: 479 (2014) | doi:10.1038/bonekey.2013.213 © 2014 International Bone & Mineral Society All rights reserved 2047-6396/14 Hector F DeLuca
Department of Biochemistry, University of Wisconsin-Madison, Madison, WI, USA. deluca@biochem.wisc.edu
Before the twentieth century, it was not possible to describe the essentials of a diet that could support life, growth and reproduction of higher animals. The discovery of vitamin A by McCollum and Davis in 1913 ushered in the era of accessory food substances culminating in the achievement of that goal. It included the discovery of vitamin D and its production in skin caused by ultraviolet light. This was followed by a description of its actions at the physiological level that resulted in a healthy skeleton and beyond. To carry out these functions, vitamin D is converted to a hormone that acts through a nuclear receptor. The findings leading to this concept and their importance to biology and medicine are presented.
Discovery of the Concept of Vitamin D
Although rickets, scurvy, beri-beri and other such diseases were known for centuries,1 the cause of them remained elusive until the twentieth century. On the basis of the dogma put forth by the influential German chemists in the nineteenth century led by von Liebig,2 an adequate diet consisted of 12% protein, 5% mineral, 10-30% fat and the remainder as carbohydrate. The belief that this defined an adequate diet was to survive until the early part of the twentieth century. In the meantime, several discoveries suggested that this was not true. First and foremost were the experiments carried out by Lunin,3 Magendie,4 Hopkins5 and Funk.6These investigators fed the recommended proportions of these purified dietary components to animals and found that the animals failed to survive. Clearly, something was missing from these purified materials required for survival. In addition, other findings were in support of the existence of essential micronutrients in the diet. One of the earliest discoveries was that of Eijkman7 who studied the high incidence of beri-beri among prisoners in the Dutch East Indies. These prisoners were fed predominantly a diet of polished rice. Eijkman found that providing the hulls of rice solved the beri-beri problem. Unfortunately, Eijkman concluded that polished rice possessed a toxin that was neutralized by a substance in the hulls. A colleague of Eijkman, that is, Grijns, revisited the question and correctly demonstrated that hulls contained an important and required nutrient that prevented beri-beri, but the idea of a vitamin had not yet been given birth.8
Another discovery of a substance that prevented scurvy among sailors was made by Hoist and Frohlich.9 They found that scurvy experienced by seamen could be prevented or cured by citrus fruits or a substance found therein. Yet, the idea of essential micronutrients of an organic type had yet to be conceived. The idea of vitamins was first suggested by Funk,6 who envisioned that a 'vital amine' present in foods was required for health and survival. Unknown to Funk and without evidence, this would prove to be a term that would describe the accessory food factors later to be discovered.
Professor Steven Moulton Babcock at the University of Wisconsin had long been in opposition of the German chemists' view that an adequate diet could be described by correct proportions of protein, carbohydrate, fat and salts.10 At long last, the Department of Dairy Science at the University of Wisconsin allowed Professor Babcockand his newly hired head of Agricultural Chemistry, that is, EB Hart, to carry out an experiment in the dairy herd at Wisconsin.11 They fed four groups of dairy cattle with the exact dietary proportions suggested by the German chemists, except that the entire ration was derived from a single grain, namely corn, oats, wheat or a mixture thereof. The outcome was quite dramatic. Cows fed the corn diet did very well, reproduced and were able to produce large amounts of milk, whereas those on the wheat diet did poorly and, in fact, failed to survive. The oat diet resulted in a finding intermediate between wheat and corn. The Wisconsin group correctly concluded that there were accessory food factors yet to be discovered that were responsible for the health and well-being of those animals fed the corn diet.
This led Professor Hart, Chair of Agricultural Chemistry at the University of Wisconsin, to begin a series of experiments to test this hypothesis. Professor Elmer McCollum was allowed to use a small animal model, the white rat, to study the importance of various dietary components. In a controversial move for a college of agriculture, Hart and Babcock permitted and, in fact, supported the use of the rat as an experimental animal despite the opposition that rats are considered enemies of the farm and should not be allowed in a College of Agricultural and Life Sciences. With the white rat, McCollum and Davis12 conclusively demonstrated that butter fat and cod liver oil contained a factor, which is required for the prevention of xerophthalmia, an eye disease, and to support growth. This finding attracted Osborne and Mendel13 at Yale to carry out similar experiments, and, independently, McCollum et a/.14 at Wisconsin and Osbourne and Mendel13 at Yale discovered a water-soluble factor that was responsible for preventing a neurological disease similar to beri-beri. McCollum, in consultation with Professor Harry Steenbock, who was also involved in the early, single-grain experiment, decided that they would use the idea of Funk to call these substances 'vitamins'. Vitamin A was the fat-soluble factor and vitamin B was the water-soluble factor. Soon thereafter, evidence was provided that another water-soluble factor prevented the disease scurvy and was called vitamin C.15 The stage was set then for the discovery of the next vitamin, vitamin D.
Discovery of Vitamin D
Sir Edward Mellanby in Great Britain had been very concerned with the extremely high incidence of rickets in the United Kingdom, especially in Scotland. In fact, the disease became known as'the English Disease'.16 Sir Mellanby was taken by the work of McCollum and decided that rickets might be a dietary deficiency disease. He very cleverly used the diet consumed by the Scottish people (who had the highest incidence of rickets), primarily oatmeal, and fed that to dogs that he inadvertently kept indoors and away from sunlight. They developed rickets, which was identical to the human disease.17 Sir Mellanby17 could cure the disease by providing cod liver oil and he therefore assumed that it was possible that vitamin A was responsible for the prevention of rickets. McCollum who had since left Wisconsin and moved to Johns Hopkins University had been following this finding, and decided to test the hypothesis of whether vitamin A was responsible for healing rickets. He bubbled oxygen through cod liver oil that destroyed vitamin A and found that this preparation was no longer able to prevent xerophthalmia and vitamin A deficiency, but it still retained the ability to cure rickets.18 McCollum et a/.18 correctly concluded that the factor that cures rickets is a new vitamin, which they called vitamin D.
Healing of Rickets by UV Light
In the meantime, Huldshinsky,19 a physician in Vienna, and Chick et a .20 in England found that children suffering from rickets could be cured by exposing them to summer sunlight or artificially produced UV light. Hess and Unger21 also noted that sunlight could cure rickets. This dichotomy attracted Professor Harry Steenbock at the University of Wisconsin who had been assigned the small animal experimental work. Steenbock in 1916 had been working with goats when he found that when they were kept in summer sun outdoors, they were in positive calcium balance but when kept indoors in the winter in the absence of sunlight, they went into negative calcium balance.22 Steenbock had then mentally made a connection between sunlight and calcium retention. With this background, Steen-bock23,24 began to irradiate rats, their food and the air in their cages with UV light. He found that irradiation of not only the rat but also their food could prevent or cure rickets. He found this activity to be associated with the non-saponifiable lipid fraction and correctly concluded that an inactive lipid in the diet and skin could be converted by UV light into an active antirachitic substance.25 Professor Steenbock26 patented the process, and with this patent was able to attract industry to use this discovery to eliminate rickets as a major medical problem. Hess and Weinstock27 independently and somewhat later discovered that irradiation could prevent rickets.
Isolation and Identification of Vitamin D
Although the idea of vitamin D became very clear and it was found in a non-saponifiable fraction, the actual identification of the vitamin structure was not to take place until 1932 when Askew eta/.28 were able to isolate vitamin D2 from an irradiation mixture of ergosterol. Vitamin D1 had proved to be an artefact of an adduct between vitamin D2 and lumisterol by Windaus and Linsert.29 Thus, vitamin D2 proved to be the first vitamin D to be isolated and identified.
In 1935, 7-dehydrocholesterol was isolated by Windaus et a/.30 and vitamin D3 was identified in 1937 by the Windaus and Bock.31 Vitamin D3 is the natural form of vitamin D formed in the skin as a result of UV irradiation of 7-dehydrocholesterol. This then raised the question of whether vitamin D is a true vitamin or whether it is normally produced in the skin and is not found in natural foods. Although it was surmised that vitamin D3 arises in skin via the irradiation of 7-dehydrocholesterol, this was not proven until 1978 when Esvelt eta/.32 actually isolated and identified vitamin D3 by mass spectrometry. Before this, Holick eta/.33 provided evidence that previtamin D3 is formed in the skin on UV irradiation. The actual chemistry of the irradiation process was defined by the work of Velluz et a/.34 and also by the contributions of Havinga.35
Figure 1 illustrates the conversion of 7-dehydrocholesterol to vitamin D3 via its intermediate previtamin D3.
Early Understanding of the Function of Vitamin D
Following these monumental discoveries, rickets disappeared as a major medical problem and the vitamin D research settled into a very quiescent state with only an occasional new discovery being made. Great strides were made, however, in the area of the water-soluble factors of vitamin B, which were then shown to be composed of several different factors, all providing different functions in the body with many assuming activated forms or coenzymes for function. Although there were attempts to describe vitamin D as a coenzyme, this proved to be a dead end.36 Thus, it was assumed from this point on that vitamin D itself functioned without metabolic change, an idea that was to persist until 1968.37
The discovery of vitamin D resulted in a variety of attempts to understand how this steroid might result in the healing of rickets and its adult counterpart, osteomalacia. One of the early experiments that is often unappreciated are the studies of Shipley et a .38,39 in which slices of bone taken from rachitic animals were incubated in the blood serum of vitamin D-deficient animals or in the blood serum of animals provided with vitamin D.
Figure 1 The conversion of 7-dehydrocholesterol to previtamin D3 by 282-310 nm UV light and the temperature-dependent equilibrium between previtamin D3 and vitamin D3.
The provisional deposit of calcium and phosphate was found in the case of bone slices incubated in serum of animals that had been given vitamin D. However, the addition of vitamin D to the serum of vitamin D-deficient rats did not in any way influence the deposit of mineral in the bone. Of considerable importance was that calcification of rachitic bone could be achieved by incubation in solutions that contained the same levels of calcium and phosphate as is found in the serum of animals given vitamin D. These results did not really indicate the mechanism of action of vitamin D, but it did suggest that the failure of mineralization might well be a failure of supply of calcium and phosphorus to the bone compartment in the case of vitamin D deficiency. This idea was to be left uninvestigated for some time.
The next important advance took place with the discovery by Nicolaysen eta/.40,41 that vitamin D increases the absorption of calcium from the small intestine. This clearly showed that vitamin D was an important factor in the utilization of dietary calcium. Nicolaysen eta/.41 also noted that animals on a low-calcium diet had much greater efficiency of calcium absorption than animals fed an adequate amount of calcium. In addition to the role of vitamin D in calcium absorption, Nicolaysen41 put forth the idea of an 'endogenous' factor that would inform the intestine of the bone requirements for calcium. Thus, under circumstances of low mineralization, bone would signal the intestine that additional calcium absorption was required. The 'endogenous' factor of Nicolaysen was clearly defined with the discovery of the functional metabolism of vitamin D described later.
In 1952, a somewhat unappreciated but major discovery was made by Carlsson42 and Bauer et a/. ,43 who found that vitamin D, rather than directly causing a deposit of mineral in bone, actually caused the mobilization of calcium from the bone into the plasma compartment. Although this would appear to decalcify the bone, it represented an important mechanism whereby vitamin D has an important role in the maintenance of serum calcium that is required not only for mineralization of the skeleton but also for neuromuscular function. Nevertheless, this discovery defined a new way that vitamin D could cause an increase in serum calcium.
Turning back to the work of McCollum eta/.44 and Steenbock and Black,25 the production of rickets in rats required not a low-calcium diet but rather a high-calcium and low-phosphorus diet. The rachitic epiphyseal plate could only be found when a high-calcium, low-phosphorus vitamin D-deficient diet was fed in the case of rats. In these animals provided vitamin D, not only was there an increase in serum calcium but also an increase in serum phosphorus.45 It was later demonstrated that a major role of vitamin D is to increase the transport of phosphate from the lumen of the intestine to the serum, providing an increase in serum phosphorus. In a further attempt to define the mechanism of action of vitamin D at the cellular, if not molecular, level, Schachter and Rosen46 introduced the idea of studying the transport of calcium in vitro using an everted sac technique. These investigators could show that vitamin D increased the active transport of calcium against a concentration gradient from the lumen of the intestine into the plasma compartment,47 underscoring the findings of Nicolaysen and indicating that this transport is an active process; one that was later confirmed by Walling and Rothman48 using classical electrophysiology methods. Phosphate absorption from the intestine is also an active process dependent on calcium, which is vitamin D dependent.49,50
Another important advance was the position taken by Lamm and Neuman51 in their physical/chemical considerations of mineralization. Lamm and Neuman51 provided evidence that the blood is normally supersaturated with calcium and phosphorus, and that mineralization is actually a catalyzed crystallization process. The idea then evolved that vitamin D increases serum calcium and phosphorus to supersaturating levels that are responsible for normal mineralization of the bone.52 A final demonstration of this was carried out by Underwood and DeLuca53 in which infusion of calcium and phosphate to maintain normal serum levels of calcium and phosphate in vitamin D-deficient rats resulted in normal mineralization. Studies then turned to an understanding of the mechanism of action of vitamin D on the intestine, bone and kidney, where calcium and phosphorus are absorbed or resorbed or mobilized. Peculiarly, rickets can be produced in rats only with a low-phosphorus, vitamin D-deficient diet, whereas a low-calcium, vitamin D-deficient diet results in a type of osteoporosis.25,45,54 However, both low serum calcium and low serum phosphorus produce rickets in humans.55,56
The Functional Metabolism of Vitamin D
A study of the time course of calcium transport by the everted sac technique by the DeLuca laboratory57 showed that a 16-h lag between dosing vitamin D3 to vitamin D-deficient animals occurred before the active transport of calcium occurred in the intestine. The same could be said for the mobilization of calcium from the bone.57 Thus, the lag signaled some important events were taking place before the action of vitamin D could be observed on calcium.
During the 1950 era, Professor Egon Kodicek of Cambridge became very interested in following what happens to the vitamin D2 molecule in the course of its function.37 Professor Kodicek laboriously prepared radiolabeled vitamin D2 by incubating yeast in 14C containing acetate medium. He was successful in obtaining vitamin D2 of fairly low specific activity to begin his studies on metabolism. Kodicek37 had attempted to study metabolism without the use of radiolabels and found it laborious and difficult to interpret. After more than a decade of experimentation, Kodicek58 concluded that vitamin D was active directly without metabolic change, as all metabolites were found without biological activity. Unfortunately, Professor Kodicek was required to use very large doses of vitamin D2 beyond what was considered a physiologic level. As a result, his studies were primarily directed to the storage of vitamin D rather than function. To obtain evidence on the lag in vitamin D activity between the time of dosing and calcium transport, it was essential to learn whether the vitamin D actually reached the intestine as quickly as expected or whether there was some delay in transport and deposit. This required the synthesis of radiolabeled vitamin D of high specific activity, which was first achieved by the Wilzbach method,59 and even more efficiently with a radiochemical synthesis putting the tritium in the 1 and 2 position, giving high-enough specific activity to follow a truly physiologic dose.60 This allowed the detection of metabolites of vitamin D very quickly (within 1-2 h) after dose and long before the intestine and bone responded.61-63 These metabolites could easily be found following Silica chromatography.61-63 The largest metabolite fraction was termed 'peak 4,' and when this was given back to vitamin D-deficient animals it proved to be more potent and acted more quickly than vitamin D3 in turning on intestinal calcium transport.61,62 In the meantime, Haussler and Norman64 published a paper using the Wilzbach-labeled vitamin D3 and concluded that vitamin D3 was active in the intestine without metabolic change. To generate large amounts of the peak 4 metabolite for identification, four pigs were given large doses of vitamin D3, and their blood plasma extracted and chromatographed several times on Silica; however, even at this state of purification, mass spectrometry was not possible because of the interfering lipids. The final step on reverse-phase chromatography was devised that produced a pure metabolite, which was clearly identified by mass spectrometry, UV absorption spectrometry and nuclear magnetic resonance spectrometry as 25-hydroxyvitamin D3 (25-OH-D3).65 This compound was quickly synthesized66 and its biological effectiveness demonstrated, as well as its rapid action.67 The synthesis of this compound from the 25-keto cholesterol material available from commercial sources could be converted to the 25-OH-D3, which allowed for the introduction of tritium in the 26 and 27 positions by a Grignard reaction.68 When this highly radiolabeled 25-OH-D was administered, it was rapidly metabolized to still other metabolites, of which two out of three found in the blood were identified.69,70 However, to be certain that the metabolically active form of vitamin D was isolated and identified, the Wisconsin group used vitamin D-deficient chickens given radiolabeled vitamin D3 in order to allow following of the metabolite. From 1600 chick intestines, a metabolite was isolated following 8 chromatographic steps to a substance, which was not yet quite pure enough for mass spectro-metry.71,72 It still contained a contaminant that interfered with identification. A final step was introduced in which all hydroxyls could be converted to a trimethylsilyl (TMS) derivative. The TMS derivative was subjected to mild acid hydrolysis, which removed the TMS from the secondary hydroxyls, leaving the 25-TMS (a tertiary hydroxyl TMS) intact. This provided a chromatographic difference between the metabolite and the contaminant, resulting in a pure 25-TMS derivative of the metabolite. By means of mass spectrometry and chemical reactions, it was identified as 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3).71,72 This structure was reported at the Parathyroid Conference in North Carolina and was the first identification of this metabolite.73 Subsequently, a report appeared from the Kodicek and colleagues74 in which a 30% pure material was reputed to have been identified as 1,25-(OH)2D3, although it was not clear how that identification could take place with such an impure preparation. Still another report appeared later but did not provide the necessary information that would allow deduction of the structure.75 The configuration of the hydroxyl on the 1 position could not be deduced until chemical synthesis was carried out, in which the 1a- and 1 p-25-dihydroxyvitamin D3 compounds could be produced.76 Byco-chromatography, it became clear that the active metabolite is 1a,25-(OH)2D3. The 1a,25-(OH)2D3 proved to be extremely potent but very short lived, and is well recognized as the metabolically active form of vitamin D in managing serum calcium, serum phosphorus and mineralization of the skeleton.57,77 The Wisconsin group continued to isolate and identify metabolites until ^30 metabolites had been identified.78 The most notable substance that required a great deal of attention was the 24R,25-dihydroxyvitamin D3, which was originally identified as 21,25-dihydroxyvitamin D370 but was later corrected by the Wisconsin group.79 Many claims have been made for this substance as an active metabolite and Boyle et a/.80 did extensive experiments in the rat to try to find a functional role for this substance. A final blow to the idea that 24,25-dihydroxyvitamin D3 as an active form was done by the synthesis of 24,24-difiuoro-25-hydroxyvitamin D3. Animals supported for two generations on this form of vitamin D that cannot be 24-hydroxylated showed no deficiency phenotype whatsoever and, in fact, fully normal and fully able to carry out reproduction and development.81 Thus, it became very clear that vitamin D must first be metabolized to a blood form, 25-OH-D3, that in itself is not directly biologically active, but be further metabolized to 1,25-(OH)2D3 to carry out its functions in calcium, phosphorus and bone metabolism57,82,83 (Figure 2). The active metabolites of vitamin D2, that is, 25-hydroxyvitamin D2 and 1,25-dihydroxyvitamin D2, were also isolated and identified.84,85
Figure 2 The functional metabolism of vitamin D3. A CYP2R1 enzyme in the liver converts vitamin D3 to 25-OH-D3, the circulating form of vitamin D3, and a CYP27B1 enzyme converts it to the 1,25-(OH)2D3 in the proximal convoluted tubule of the kidney to the final hormone, 1a,25-(OH)2D3.
The Vitamin D Endocrine System
Following identification of 25-OH-D3, the organ responsible for the conversion of vitamin D3 to 25-OH-D3 was determined to be the liver because subtotal hepatectomy largely eliminated this conversion.86 The enzyme largely responsible is the CYP2R1 as will be described in subsequent chapters.87
Fraser and Kodicek88 demonstrated that the conversion of 25-OH-D3 to what was later identified by Holick et a/.71,72 as 1,25-(OH)2D3 takes place in the kidney and, in particular, in the proximal convoluted tubule.89 It was cloned as the CYP27B1 by three groups.90-92
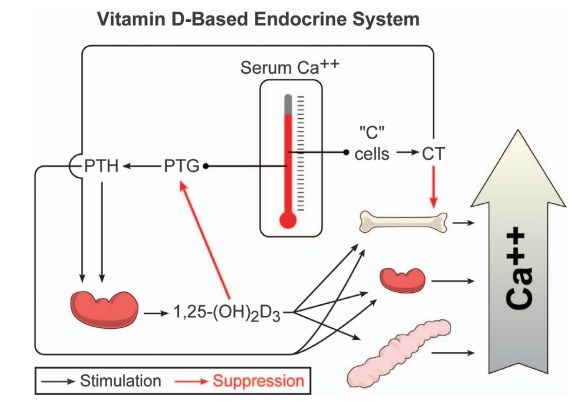
Figure 3 A diagrammatic representation of the vitamin D-based endocrine system. The calcium-sensing proteins in the parathyroid and 'C cells are shown as a thermometer. Slight hypocalcemia causes secretion of PTH that signals the CYP27B1 to synthesize 1,25-(OH)2D3 that directs calcium mobilization in the intestine, kidney and bone. A feedback suppression of PTH synthesis and secretion, and parathyroid proliferation by 1,25-(OH)2D3 is shown. Calcitonin is secreted by the 'C cells of the thyroid. It suppresses bone resorption.
With the advent of molecular biology, transcripts of the CYP27B1 have been found in several cells not previously recognized as a site of production of 1,25-(OH)2D3. Reports of the existence and the generation of 1,25-(OH)2D3 in small amounts in these tissues have suggested a paracrine/autocrine function of the vitamin D system. This is likely true but has not been firmly established by rigorous chemical methods. In any case, subsequent chapters will discuss the possible paracrine/ autocrine functions of the vitamin D system.
During the course of this development, Ian Boyle in the DeLuca laboratory became very interested in the regulation of the metabolism of vitamin D. In a classical paper, it was demonstrated that when a low-calcium diet is fed a high degree of conversion of 25-OH-D3 to 1,25-(OH)2D3 occurs, giving very high blood levels of 1,25-(OH)2D3, whereas when high-calcium diets are fed the synthesis of the active form of vitamin D is clearly suppressed producing low amounts.93,94 The beginning of a feedback regulation system became clear. Garabedian eta/.95 was able to show that hypocalcemia was detected by the parathyroid glands and, in response, the parathyroid hormone (PTH) proved to be the stimulus of the 1 a-hydroxylase in the kidney to produce 1,25-(OH)2D3. When the parathyroids were removed, the animal was unable to sense the hypocalcemia and interpret it by producing 1,25-(OH)2D3. Although the exact mechanism has yet to be deduced, it is clear that parathyroid acts through the G-protein mechanism to stimulate the transcription and translation of the 1 a-hydroxylase enzyme to produce 1,25-(OH)2D3, and this still remains as the major regulator of synthesis of this very potent calcium-mobilizing hormone.96 Nicolaysen's 'endogenous factor' was clearly identified as the PTH/1,
25-(OH)2D system.52,94
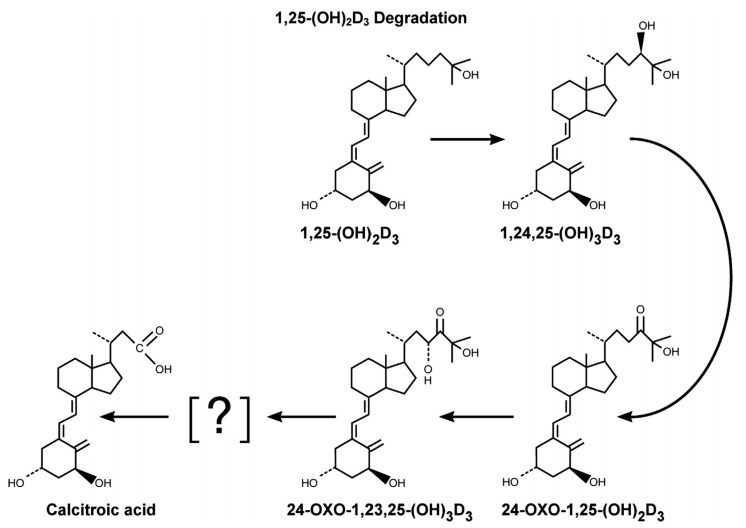
Figure 4 Degradation of 1 a,25-(OH)2D3. The CYP24A1 gene is induced by 1,25-(OH)2D3.
The resulting enzyme carries out all the reactions shown to produce the biologically inactive excretion product, calcitroic acid.
Presumably, a similar set of reactions takes place with 25-OH-D3 as the substrate. Clearly, 1,25-(OH)2D3 programs its own destruction through the CYP24A1.
Attention was then focused on the 24-hydroxylase and its importance in the vitamin D endocrine system. This enzyme, which is a cytochrome P-450, is located in kidney mitochondria and in all target tissues of the vitamin D hormone.96-99 The enzyme has been cloned and a knockout has been produced.100 The intermediates in the metabolism of 1,25-(OH)2D3 and the final product, calcitroic acid, have clearly been identified96,98,101 and is the primary excretory product of the active form of vitamin D. Recently, human patients have been identified with inactivating mutations of CYP24A1, elevations of serum 1,25-(OH)2D3 and idiopathic infantile hypercalcemia, further supporting a catabolic function for CYP24A1.102 Figure 3 illustrates the known conversion of 1,25-(OH)2D3 to its excretion product, calcitroic acid. Thus, it became clear that in vitamin D deficiency, CYP24A1 is not expressed and is induced by the vitamin D hormone acting through its receptor.103 Thus, the active form of vitamin D induces its own destruction by turning on the synthesis of the enzyme that metabolizes it to an inactive final product. CYP24A1 also acts on 25-OH-D to produce 24,25-(OH)2D3 and is still believed to be a route of elimination of 25-OH-D. Thus far, no functional importance beyond this has been found for this important metabolite of vitamin D.
An important chapter to the vitamin D endocrine system is one that involves a feedback regulation of the parathyroid gland (Figure 4). The parathyroid gland has a very high concentration of the vitamin D receptor.104 It is very clear from the work of two different groups that 1,25-(OH)2D3 through its receptor suppresses the preproparathyroid gene and diminishes the production and secretion of the pth.105,106 This is an important mechanism that has a striking role in the development of the disease, renal osteodystrophy. It is this target that is used to treat the secondary hyperpara-thyroidism of patients on dialysis, who have lost the ability to produce the vitamin D hormone. Its success in this capacity is clearly shown by not only its use to suppress secondary hyperparathyroidism but the development of at least two or three important analogs of vitamin D that are sold commercially for this purpose.107-109 Figure 4 puts together the vitamin D endocrine system and its regulation. In subsequent chapters, various aspects of this endocrine system will be discussed. Notably absent from this figure is the fibroblast growth factor-23 involvement with vitamin D metabolism. This discussion is beyond the scope of this historical chapter and will be covered by subsequent chapters.
From 1965 until 1975, the elements of the vitamin D endocrine system that regulate calcium and phosphorus became clear. A great deal of work remains to be done to understand how this system works, what are the many additional regulators that can be found and how does it work in regulating the transcription and suppression of target genes. These advances so far have provided a number of important forms of vitamin D for the treatment of disease and important new insights into the etiology of those diseases.
Conflict of Interest: The author declares no conflict of interest.
Acknowledgements: This work was supported by a fund from the Wisconsin Alumni Research Foundation.
References
1. McCollum EV. A History of Nutrition. The Riverside Press: Cambridge, MA, 1957, pp 234-236.
2. von Liebig J. Animal chemistry or organic chemistry in its application to physiology and pathology. In: Glass HB (ed). A History of Nutrition. Cambridge, MA: The Riverside Press, 1957.
3. Lunin N. Uber die bedeutung der anorganischen salze fur die ernahrung des tieres. ZPhysiol Chem 1881;5:31-39.
4. Magendie F. Ann. Chim. Phys. 1816; 3: 86-87. In: Glass HB (ed). A History of Nutrition by McCollum EV. The Riverside Press: Cambridge, MA, 1957.
5. Hopkins G. Feeding experiments illustrating the importance of accessory food factors in normal dietaries. J Physiol 1912;44:425-460.
6. Funk C. The chemical nature of the substance that cures polyneuritis in birds produced by a diet of polished rice. J Physiol (London) 1911;43:395-402.
7. Eijkman C. Ein beri-beri anliche der huhner. Virchows Arch 1897;148:523-527.
8. Grijns J. Research on vitamins 1900-1911. J Noordyn Gorinchem 1935;37:38.
9. Hoist A, Frohlich T. Experimental studies relating ship-beriberi to scurvy. II. On the etiology of scurvy. JHyg 1907;7:634-671.
10. de Kuif P. Hunger Fighters. Harcourt, Brace and Company: New York, 1928.
11. Hart EB, McCollum EV, Steenbock H, Humphrey GC. Physiological effect on growth and reproduction of rations balanced from restricted sources. Wis Agric Exp Sta Res Bull 1911;17:131-205.
12. McCollum EV, Davis M.The necessity of certain lipins in the diet during growth. J Biol Chem 1913;25:167-175.
13. Osborne TB, Mendel LB. The role of vitamins in the diet. J Biol Chem 1917;31:149-163.
14. McCollum EV, Simmons N, Pitz W. The relation of the unidentified dietary factors, the fat-soluble A and water-soluble B of the diet to the growth promoting properties of milk. J Biol Chem 1916;27:33-38.
15. Drummond JC. The nomenclature of the so-called accessory food factors (vitamins). Biochem J 1920;14:660.
16. Hess A. The history of rickets. In: Rickets, Including Osteomalacia and Tetany. Lea & Febiger: Philadelphia, 1929, pp 22-37.
17. Mellanby E. An experimental investigation on rickets. Lancet 1919;1:407-412.
18. McCollum EV, Simmonds N, Becker JE, Shipley PG. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem 1922; 53:293-298.
19. Hulshinsky K. Heilung von rachitis durch kunstlich hohen-sonne. Deut. Med. Wochenscher. 1919; 45: 712-713; Z. Orthopad. Chir. 1920; 39:426 as described in Bills CE. In: Sebrell Jr WH, Harris RS (eds). The Vitamins. Vol. II. Academic Press: New York, 1954, pp 162.
20. Chick H, Palzell EJ, Hume EM. Studies of rickets in Vienna 1919-1922. Medical Research Council, Special Report: No. 77, 1923.
21. Hess AF, Unger LL. The cure of infantile rickets by sunlight. JAMA 1921;77:39-41.
22. Steenbock H, Hart EB. The influence of function on the lime requirements of animals. J Biol Chem 1913;14:59-73.
23. Steenbock H. The induction of growth promoting and calcifying properties in a ration by exposure to light. Science 1924;60:224-225.
24. Steenbock H, Black A. Fat-soluble vitamins. XVII. The induction of growth-promoting and calcifying properties in a ration by exposure to ultraviolet light. J Biol Chem 1924; 61 :405-422.
25. Steenbock H, Black A. Fat-soluble vitamins. XXIII. The induction of growth-promoting and calcifying properties in fats and their unsaponifiable constituents by exposure to light. J Biol Chem 1925;64:263-298.
26. Steenbock H. US Patent Number 1680818 (application, 1924; issued 1928).
27. Hess A, Weinstock M. Antirachitic properties imparted to lettuce and to growing wheat by ultraviolet irradiation. Proc Soc Exp Biol Med 1924;22:5-6.
28. Askew FA, Bourdillon RB, Bruce HM, Jenkins RGC, Webster TA. The distillation of vitamin D. Proc R Soc 1931;B107:76-90.
29. Windaus A, Linsert O. Vitamin D1. Ann Chem 1928;465:148.
30. Windaus A, Lettre H, Schenck F. 7-dehydrocholesterol. Ann Chem 1935;520:98-107.
31. Windaus A, Bock F. Uber das provitamin aus dem sterin der schweineschwarte. Z Physiol Chem 1937;245:168-170.
32. Esvelt RP, Schnoes HK, DeLuca HF. Vitamin D3from rat skins irradiated in vitro with ultraviolet light. Arch Biochem Biophys 1978;188:282-286.
33. Holick MF, FrommerJE, McNeill SC, Richtand NM, HenleyJW, Potts JrJT. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res Commun 1977;76:107-114.
34. Velluz L, Amiard G, Petit A. Le precalciferol-ses relations d'equilibre avc le calciferol. Bull Soc Chim Fr 1949;16:501-508.
35. Havinga E. Vitamin D, example and challenge. Experientia 1973;29:1181-1193.
36. Zetterstrom R. Phosphorylation of vitamin D2, and the action of the phosphorylated compound on alkaline kidney phosphatase. Nature 1951;167:409-410.
37. Kodicek E. Metabolic studies on vitamin D. In: Wolstenholme GWE, O'Connor CM (eds). Ciba Foundation Symposium on Bone Structure and Metabolism. Little, Brown and Co: Boston, 1956, pp 161-174.
38. Shipley PG, Kramer B, Howland J. Calcification of rachitic bones in vitro. Am J Dis Child 1925; 30:37-39.
39. Shipley PG, Kramer B, Howland J. Studies upon calcification in vitro. Biochem J 1926; 20:379-387.
40. Nicolaysen R. Studies upon the mode of action of vitamin D. III. The influence of vitamin Don the absorption of calcium and phosphorus in the rat. Biochem J 1937;31:122-129.
41. Nicolaysen R, Eeg-Larsen N, Malm OJ. Physiology of calcium metabolism. Physiol Rev 1953;33:424-444.
42. Carlsson A. Tracer experiment on the effect of vitamin D on the skeletal metabolism of calcium and phosphorus. Acta Physiol Scand 1952;26:212-220.
43. Bauer GCH, Carlsson A, Lindquist B. [Evaluation of accretion, resorption and exchange reactions in the skeleton]. Kungl Fysiograf Sallskap I Lund Forh 1955;25: 3-18.
44. McCollum EV, Simmons N, Becker JE, Shipley PG. Studies on experimental rickets. XXVI. A diet composed principally of purified foodstuffs for use with the 'line test' for vitamin D studies. J Biol Chem 1925; 65:97-100.
45. Cramer JW, Steenbock H. Calcium metabolism and growth in the rat on a low phosphorus diet as affected by vitamin D and increases in calcium intake. Arch Biochem Biophys 1956; 63:9-13.
46. Schachter D, Rosen SM. Active transport of Ca45 by the small intestine and its dependence on vitamin D. Am J Physiol 1959;196:357-362.
47. Dowdle EB, Schachter D, Schenker H. Requirement for vitamin D for the active transportof calcium by the intestine. Am J Physiol 1960;198:269-274.
48. Walling MW, Rothman SS. Phosphate-independent, carrier-mediated active transport of calcium by rat intestine. Am J Physiol 1969;217:1144-1148.
49. Chen TC, Castillo L, Korycka-Dahl M, DeLuca HF. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr 1974;104:1056-1060.
50. Lee DB, Walling MW, Corry DB. Phosphate transport across rat jejunum: Influence of sodium, pH, and 1,25-dihydroxyvitamin D3. Am J Physiol 1986;251:G90-G95.
51. Lamm M, Neuman WF. On the role of vitamin D in calcification. Arch Pathol 1958;66:204-209.
52. DeLuca HF. Mechanism of action and metabolic fate of vitamin D. Vitam Horm 1967; 25:315-367.
53. Underwood JL, DeLuca HF. Vitamin D is not directly necessary for bone growth and mineralization. Am J Physiol 1984;246:E493-E498.
54. Steenbock H, Herting DC. Vitamin D and growth. J Nutr 1955;57:449-468.
55. Jones JH. VI. Effects of deficiency. In: Sebrell Jr WH, Harris RS (eds). The Vitamins Vol. II. Academic Press: New York, 1954, pp 223-232.
56. Kramer B, Kanof AB. In pathology of human beings. In: Sebrell Jr WH, Harris RS (eds). The Vitamins. Vol. II. Academic Press: New York, 1954, pp 232-248.
57. DeLuca HF. Vitamin D: the vitamin and the hormone. Fed Proc 1974;33:2211-2219.
58. Kodicek E. The metabolism of vitamin D. In: Umbreit W, Molitor H (eds). Proceedings of the Fourth International Congress of Biochemistry 1960;XI:198-208.
59. Norman AW, DeLuca HF. The preparation of H3-vitamin D2 and D3 and their localization in the rat. Biochemistry 1963;2:1160-1168.
60. Neville PF, DeLuca HF. The synthesis of [1,2-3H] vitamin D3 and the tissue localization of a 0.25 mg (10 IU) dose per rat. Biochemistry 1966;5:2201-2207.
61. Lund J, DeLuca HF. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res 1966;7:739-744.
62. Morii H, Lund J, Neville PF, DeLuca HF. Biological activity of a vitamin D metabolite. Arch Biochem Biophys 1967;120:508-512.
63. Norman AW, Lund J, DeLuca HF. Biologically active forms of vitamin D3 in kidney and intestine. Arch Biochem Biophys 1964;108:12-21.
64. Haussler MR, Norman AW. The subcellular distribution of physiological doses of vitamin D3. Arch Biochem Biophys 1967;118:145-153.
65. Blunt JW, DeLuca HF, Schnoes HK. 25-Hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 1968;7:3317-3322.
66. Blunt JW, DeLuca HF. The synthesis of 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 1969;8:671-675.
67. Blunt JW, Tanaka Y, DeLuca HF. The biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc Natl Acad Sci USA 1968;61:1503-1506.
68. Suda T, DeLuca HF, Hallick RB. Synthesis of [26,27-3H]-25-hydroxycholecalciferol. Anal Biochem 1971;43:139-146.
69. Suda T, DeLuca HF, Schnoes HK, Tanaka Y, Holick MF. 25,26-dihydroxycholecalciferol, a metabolite of vitamin D3 with intestinal calcium transport activity. Biochemistry 1970; 9:4776-4780.
70. Suda T, DeLuca HF, Schnoes HK, Ponchon G, Tanaka Y, Holick MF. 21,25-Dihydrox-ycholecalciferol. A metabolite of vitamin D3 preferentially active on bone. Biochemistry 1970;9:2917-2922.
71. Holick MF, Schnoes HK, DeLuca HF. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc Natl Acad Sci USA 1971;68: 803-804.
72. Holick MF, Schnoes HK, DeLuca HF, SudaT, Cousins RJ. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry 1971;10:2799-2804.
73. DeLuca HF. The functional metabolism of vitamin D3. In: Talmage RV, Munson PL (eds). Calcium, Parathyroid Hormone and the Calcitonins: Proceedings of the Fourth Parathyroid Conference. Excerpta Medica: Amsterdam, 1972, pp 221-235.
74. Lawson DEM, Fraser DR, Kodicek E, Morris HF, Williams DH. Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature 1971; 230:228-230.
75. Norman AW, Myrtle JF, Midgett RJ, Nowicki HG, Williams V, Popjak G. 1,25-Dihydrox-ycholecalciferol: Identification of the proposed active form of vitamin D3 in the intestine. Science 1971;173:51-54.
76. Semmler EJ, Holick MF, Schnoes HK, DeLuca HF. The synthesis of 1a,25-dihydrox-ycholecalciferol—a metabolically active form of vitamin D3. Tetrahedron Lett 1972;40: 4147-4150.
77. Tanaka Y, Frank H, DeLuca HF. Biological activity of 1,25-dihydroxyvitamin D3 in the rat. Endocrinology 1973;92:417-422.
78. DeLuca HF, Schnoes HK. Vitamin D: recent advances. Annu Rev Biochem 1983; 52:411-439.
79. Holick MF, Schnoes HK, DeLuca HF, Gray RW, Boyle IT, SudaT. Isolation and identification of 24,25-dihydroxycholecalciferol: a metabolite of vitamin D3 made in the kidney. Biochemistry 1972;11:4151-4155.
80. Boyle IT, Omdahl JL, Gray RW, DeLuca HF. The biological activity and metabolism of 24,25-dihydroxyvitamin D3. J Biol Chem 1973;248:4174-4180.
81. Brommage R, DeLuca HF. Evidence that 1,25-dihydroxyvitamin D3 is the physiologically active metabolite of vitamin D3. Endocrine Rev 1985;6:491-511.
82. Boyle IT, Miravet L, Gray RW, Holick MF, DeLuca HF. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology 1972; 90:605-608.
83. Holick MF, Garabedian M, DeLuca HF. 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science 1972;176:1146-1147.
84. Suda T, DeLuca HF, Schnoes H, Blunt JW. 25-Hydroxyergocalciferol: A biologically active metabolite of vitamin D2. Biochem Biophys Res Commun 1969;35:182-185.
85. Jones G, Schnoes HK, DeLuca HF. Isolation and identification of 1,25-dihydroxyvitamin D2. Biochemistry 1975;14:1250-1256.
86. Ponchon G, Kennan AL, DeLuca HF. 'Activation' of vitamin D by the liver. J Clin Invest 1969;48:2032-2037.
87. Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: A microsomal vitamin D 25-hydroxilase. J Biol Chem 2003;278:38084-38093.
88. Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biologically active vitamin D metabolite. Nature 1970;228:764-766.
89. Brunette MG, Chan M, Ferriere C, Roberts KD. Site of 1,25-dihydroxyvitamin D3 synthesis in the kidney. Nature 1978;276:287-289.
90. Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T et al. Cloning and expression of rat 25-hydroxyvitamin D3-1a-hydroxylase cDNA. Proc Natl Acad Sci USA 1997;94:12920-12925.
91. Takayama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3-1a-hydroxylase and vitamin D synthesis. Science 1997;277:1827-1830.
92. St Arnaud R, Messerlian S, Moir JM, Omdahl JL, GlorieuxFH.The 25-hydroxyvitamin D 1a-hydroxylase gene maps to the pseudovitamin D deficiency rickets (PDDR) disease locus. J Bon Miner Res 1997;12:1552-1558.
93. Boyle IT, Gray RW, DeLuca HF. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci USA 1971;68:2131-2134.
94. Boyle IT, Gray RW, Omdahl JL, DeLuca HF. Calcium control of the in vivo biosynthesis of 1,25-dihydroxyvitamin D3: Nicolaysen's endogenous factor. In: TaylorS (ed). Endocrinology 1971, Proceedings of the Third International Symposium. Wm. Heinemann Medical Books Ltd: London, 1972, pp 468-476.
95. Garabedian M, Holick MF, DeLuca HF, Boyle IT. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci USA 1972;69:1673-1676.
96. Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev 1998;78:1193-1231.
97. Knutson JC, DeLuca HF. 25-Hydroxyvitamin D3-24-hydroxylase: subcellular location and properties. Biochemistry 1974;13:1543-1548.
98. Beckman MJ, Tadikonda P, Werner E, Prahl J, Yamada S, DeLuca HF. The human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry 1996; 35:8465-8472.
99. Omdahl JL, May B. The 25-hydroxyvitamin D 24-hydroxylase. In: Feldman D, Pike JW, Glorieux FH (eds). Vitamin D. 2nd edn Vol. 1. Elsevier Academic press: Burlington, MA, 2005, 85-104.
100. St Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K etal. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 2000;141:2658-2666.
101. Esvelt RP, Schnoes HK, DeLuca HF. Isolation and characterization of 1a-hydroxy-23-carboxytetranovitamin D: a major metabolite of 1,25-dihydroxyvitamin D3. Biochemistry 1979;18:3977-3983.
102. Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 2011;365:410-421.
103. Endres B, Kato S, DeLuca HF. Metabolism of 1a,25-dihydroxyvitamin D3 in vitamin D receptor ablated mice in vivo. Biochemistry 2000;39:2123-2129.
104. Hughes MR, Haussler MR. 1,25-Dihydroxyvitamin D3 receptors in parathyroid glands. Preliminary characterization of cytoplasmic and nuclear binding components. J Biol Chem 1978; 253:1065-1073.
105. Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 1992;89: 8097-8101.
106. Silver J, Naveh-Many T. Vitamin D and the parathyroids. In: Feldman D, Pike JW, Glorieux FH (eds). Vitamin D. 2nd edn Elsevier Academic Press: San Diego, CA, 2005, 537-549.
107. Slatopolsky E, Weerts C, Thieland J, Horst R, Harter H, Martin K. Marked suppression of secondary hyperparathyroidism by I.V. administration of 1,25-dihydroxycholecalciferol in uremic patients. J Clin Invest 1984;74:2136-2143.
108. Slatopolsky E, Finch J, Ritter C, Denda M, Morrissey J, Brown A et al. A new analog of calcitriol, 19-nor-1,25-(OH)2D2, suppresses parathyroid hormone secretion in uremic rats in the absence of hypercalcemia. Am J Kidney Dis 1995;26: 852-860.
109. Finch JL, Brown AJ, Mori T, Nishii Y, Slatopolsky E. Suppression of PTH and decreased action on bone are partially responsible for the low calcemic activity of 22-oxacalcitriol relative to 1,25-(OH)2D3.J. Bone Miner Res 1992;7:835-839.
PDF is attached at the bottom of this page
