History of vitamin D
The history of the discovery of vitamin D and its daughter steroid hormone
Ann Nutr Metab. 2012;61(3):199-206. doi: 10.1159/000343104. Epub 2012 Nov 26.
Norman AW.
Department of Biochemistry and Division of Biomedical Sciences, University of California Riverside, Riverside, Calif., USA.
It is largely through historical accident in the interval of 1920-1940 that vitamin D(3) became classified as a vitamin rather than as a steroid hormone.
The formal definition of a vitamin is that it is a trace dietary constituent required to produce the normal function of a physiological process or processes. The emphasis here is on trace and the fact that the vitamin must be supplied regularly in the diet; this implies that the body is unable to metabolically synthesize the vitamin in question.
However, the ultraviolet exposure of 7-dehydrocholesterol present in the skin results in the photochemical production of vitamin D(3).
Thus, vitamin D(3) becomes a true vitamin only when the animal or human does not have regular access to sunlight or ultraviolet light.
Under normal physiological circumstances, all mammals, including humans, can generate, via ultraviolet exposure of 7-dehydrocholesterol present in the skin, adequate quantities of vitamin D(3) to meet their nutritionally defined requirements.
There is a vibrant historical record beginning in 1650 and culminating in 1963 concerned with the determination of the chemical structures of vitamin D(3) and vitamin D(2).
A surprising aspect concerning vitamin D(3) is that it is itself biologically inert . There are no known essential biological actions or contributions that rely specifically on the molecule vitamin D(3).
While chemists had certainly appreciated the strong structural similarity between the vitamins D and other steroids, this correlation was never widely acknowledged in the biological, clinical, or nutritional sciences until 1965-1970.
The biological role of vitamin D(3) is to serve as a substrate for the liver 25-hydroxylase which produces 25-hydroxyvitamin D(3) [25(OH)D(3)].
25(OH)D(3) in turn serves as the substrate for the kidney proximal tubule 25(OH)D(3)-1α-hydroxylase enzyme which produces the steroid hormone 1α,25(OH)(2)-vitamin D(3) [1α,25(OH)(2)D(3)].
Introduction
It is largely through historical accident that vitamin D3 has been classified as a vitamin rather than as a steroid hormone. The formal definition of a vitamin is that it is a trace dietary constituent required to participate in the normal function of a specific physiological process or processes. The emphasis here is on trace and the fact that the vitamin must be supplied regularly in the diet; this implies that the body is unable to metabolically synthesize the vitamin in question. However, the ultraviolet exposure of 7-dehydrocholesterol present in the skin (fig. 1) results in the photochemical production of vitamin D3. Thus, vitamin D3 becomes a true vitamin only when the animal or human does not have regular access to sunlight or ultraviolet light. Under normal physiological circumstances, all mammals, including humans, can generate, via ultraviolet exposure of 7-dehydrocholesterol present in the skin, significant quantities of vitamin D3 to meet their nutritionally defined requirements.
Fig. 1.
Structural relationship of vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) with their respective provitamins, 7-dehydrocholesterol and ergoster-ol. The two structural representations presented at the bottom for both vitamin D3 and vitamin D2 are equivalent. Both sec-osteroids have 360 degree rotation (millions of times per second) around the carbon 6-carbon 7 single bond. Thus, both vitamin D secosteroids are highly conformationally flexible and present to their local environment a plethora of three-dimensional shapes. The only structural difference between vitamin D3 and vitamin D2 is the side chain. Vitamin D3 has the side chain of cholesterol (shown separately), while vitamin D2 has the side chain of ergosterol. Also vitamin D2 has a C22=C23 double bond and an additional methyl group on C24. It is to be emphasized that vitamin D3 is the naturally occurring form of the vitamin; it is produced from 7-dehydrocholesterol which is present in the skin by the action of sunlight. Vitamin D2 is produced commercially by the irradiation of the plant sterol ergosterol with ultraviolet light. According to R.P. Heaney, vitamin D2 has only ~30% of the biological activity of vitamin D3 in humans and only 10% in birds [48, 49].
But a surprising aspect concerning the chemical substance vitamin D3 is that it is biologically inert. There are no known essential biological actions generated specifically by the molecule vitamin D3. While chemists since the 1930s have certainly appreciated the strong structural similarity between the vitamins D and other steroid hormones, this correlation was never widely acknowledged in the biological, clinical, or nutritional sciences until it was discovered in 1965-1971 that the biological role of vitamin D3 is to serve as a substrate for the liver 25-hydroxylase which produces the product 25-hy-droxyvitamin D3 [25(OH)D3]. Then, 25(OH)D3 in turn serves as the substrate for the kidney proximal tubule 1a-hydroxylase enzyme which produces the steroid hormone 1a,25(OH)2-vitamin D3 [1 a,25(OH)2D3].
History from 1645 to 1900
The first scientific description of the classic bone disease rickets was provided in 1645-1660 by Dr. Daniel Whistler (1619-1684) at the University of Leiden, The Netherlands [1], and Prof. Francis Glisson (1597-1677) at the University of Cambridge, UK [2] .
The 18th century provided little in the way of specific advances towards the discovery of vitamin D. It can be most properly characterized as a period of recognition and acceptance of the views of Glisson and Whistler, i.e. that there was a distinct bone disease state termed rickets. In 1849, Armand Trousseau (1801-1867) and Charles Lasegue (1816-1883) [3] appreciated that osteomalacia and rickets were different expressions of the same malady, while Gustav Pommer (1851-1935) in 1885 provided a thorough histological and pathological description of the rachitic skeleton [4]. Although cod liver oil had been used medicinally for some time, it was in 1824 that D. Schiitte proposed cod liver oil as a treatment for rickets and os-teomalacia [5]. Theobald Palm, in 1890, pioneered a quantitative geographic study of the worldwide distribution of rickets, especially in all European countries, China, Japan, India, West Indies, and the United States [6].
Discovery of an Antirachitic Factor, 1901-1930
These observations on rickets set the stage for the later brilliant formulation of the vitamin concept in 1906 by Sir Frederick Gowland Hopkins (1861-1947) [7]. Also in 1914, Casimir Funk (1884-1967) wrote in his classic Die Vitamine : 'It is very probable that rickets occurs only when certain substances in the diet essential for normal metabolism are lacking or are supplied in insufficient amounts. The substances occur in good breast milk, also in cod-liver oil, but are lacking in sterilized milk and in cereals'; this was translated in 1929 by Alfred F. Hess (1875-1933) [8].
These views were not overlooked by Sir Edward Mel-lanby (1884-1955). In a landmark series of studies (from 1919 to 1924) involving the feeding of a plethora of scientifically devised diets to more than 400 dogs over a period of 5 years, he unequivocally established that rickets was caused by a deficiency of a trace component present in the diet [9, 10]. In 1921, he wrote: 'The action of fats in rickets is due to a vitamin or accessory food factor which they contain, probably identical with the fat-soluble vitamin' [11]. Furthermore, he established that cod liver oil was an excellent antirachitic agent.
Mellanby was acutely aware of the complicated nature of rickets and understood how the existence of a specific antirachitic substance could have been previously overlooked. He stated: 'It has been shown that many of the food elements exert a potent influence on the operation of bone calcification or growth.' A detailed summary of Mellanby's scientific and medical contributions and accomplishments is in 'Sir Edward Mellanby (1884-1955): The Man, Research Worker, and Statesman' by B.S. Plant in 1956 in the Annual Reviews of Biochemistry [12].
Although many would argue that the prime accomplishment of Mellanby was the unequivocal demonstration that a true dietary component was the causative agent of rickets, in retrospect, his accomplishments were much more far-reaching. Of far greater value was his application of the scientific technique to the infant field of nutrition so that it was possible to routinely raise a vitamin D-deficient animal. This great stride forward made it possible for scientists all over the world to use this scientific technique to unravel the mode of action of the elusive 'antirachitic factor'.
As with all phases of rapid development, it is difficult in retrospect to unravel the precise order of discovery of the many facets of the total problem. There were three areas in which progress had to be made: (1) separation of vitamin A and D activities; (2) appreciation that ultraviolet light and cod liver oil could both effect the same cure of rickets, and (3) demonstration that irradiation of food (in the absence of the animal) produces the same effect as irradiation of the animal.
Kurt Huldschinsky (1883-1941) [ 13] first showed in 1919 that the ultraviolet rays from a mercury vapor lamp were quite effective in increasing the calcification of the epiphysis of rachitic infants.
In their historic paper, Elmer McCollum (1879-1967) et al. [14] demonstrated that the antirachitic activity of cod liver oil could survive both aeration and heating to 100 °C for 14 h, whereas the 'anti-xerophthalmic factor', or vitamin A, was inactivated by this process. They stated:
[ The evidence set forth in this paper demonstrates that the power of certain fats to initiate the healing of rickets depends on the presence of a substance which is distinct from fat-soluble A. these experiments clearly demonstrate the existence of a fourth vitamin whose specific property, as far as we can tell at present, is to regulate the metabolism of the bones.'
Later, the new substance was named vitamin D. Although the correlation between ultraviolet light and cod liver oil in terms of their equivalent efficacy in preventing rickets was appreciated by most of the workers of that period, there was no simple explanation put forth for the observation. Both ultraviolet light and cod liver oil were found to be equivalently effective in reversing the roent-genographic evidence of the ravages of rickets upon the skeleton. However, until the separate work of Harry Goldblatt (1891-1977) and Harry Steenbock (1886-1967) no connection was made between the mysterious curative powers of ultraviolet light on rickets and the presence of an equally effective molecular species in cod liver oil.
In 1923, Goldblatt and Katherine Soames [15, 16] irradiated rat livers that had been excised from rachitic rats with ultraviolet light and found that when the irradiated tissue was ground and fed to other rachitic rats, there was a remission of the D deficiency. In parallel studies, Steenbock and Black [17] and Steenbock et al. [18] found that food which was irradiated and subsequently fed to ra-chitic rats had acquired the property of being 'antirachit-ic'. In short, both groups had for the first time produced in vitro the elusive vitamin D component of the fat-soluble vitamin. Without a doubt, the specific effect of light was no longer mysterious; it simply had produced a permanent chemical change in a component in the rat diet.
Hess and Weinstock [19, 20] in an elegant experiment confirmed the dictum that 'light equals vitamin D'. They excised a small portion of skin from rachitic rats, irradiated it with ultraviolet light, and fed the skin to groups of rachitic rats. The skin that had been irradiated provided an absolute protection against rickets, whereas the non-irradiated skin provided no protection whatsoever.
Structure Determination of Vitamins D3 and D2, 1930-1963
Beginning in 1930, a description of the evolution of our understanding of vitamin D becomes largely chemical in nature. What was unappreciated initially was that Steenbock et al. [18] had produced vitamin D2 from irradiation of the ergosterol in a yeast component of their rat diet, whereas Hess and Weinstock [19, 20] had generated vitamin D3 via irradiation of the skin. Also, the relation of both of these substances to the antirachitic component of cod liver oil remained to be established. Vitamin D3 and vitamin D2 and their respective provitamins, 7-dehy-drocholesterol and ergosterol, have both significant structural similarities and differences (fig. 1). It is important to know that ergosterol and vitamin D2 are not bio-synthesized or present in vertebrates. Thus, strictly speaking, both ergosterol and vitamin D2 are structural analogs of the naturally occurring 7-dehydrocholesterol and vitamin D3 and there should be no expectation that they have the same biological effects.
These studies began to culminate in 1932 when Adolf Windaus (1876-1959) et al. [21] and Frederick A. Askew and colleagues [22] separately but simultaneously identified the chemical structure of vitamin D2. The puzzling inability of digitonin to precipitate the 'antirachitic sterol' was now solved; the antirachitic sterol, vitamin D, was actually a secosterol. The implications of this fact were largely unappreciated for another 25-30 years.
Vitamin D3 was not chemically characterized until 1936, when Windaus et al. [23] determined the structure of the antirachitic factor that resulted after ultraviolet irradiation of 7-dehydrocholesterol. Virtually simultaneously, the elusive antirachitic component of cod liver oil was shown to be identical to vitamin D3 by Brockmann [24] in 1936. Brockmann isolated 2 g of crystalline vitamin D3 from 150,000 g of tuna liver oil. At last, all was clear. Natural vitamin D present in cod liver oil is identical to vitamin D3. Thus 7-dehyrocholesterol, not ergos-terol, is the true provitamin of the 'natural' vitamin D3.
Of equal importance, it was unmistakably clear that the antirachitic substance, vitamin D, was a steroid, more specifically a secosteroid (fig. 1). Thus, the close of the 'structure determination of vitamins D3 and D2' era of vitamin D was virtually at hand. All that remained was for Dr. Dorothy Crowfoot-Hodgkin (1910-1994) in 1948 to complete her laborious, but elegant, X-ray crystallo-graphic structural analysis of the vitamin D3 molecule [25, 26], which emphasizes the seco nature of vitamin D. Because the 9-10 carbon bond of ring B of the provitamin is broken upon irradiation, the A ring is free to assume a more extended configuration (fig. 1). However, in all other respects, the X-ray image of vitamin D3 is like that of most other steroids. Thus, the official chemical name of vitamin D3 is 9,10-secocholesta-5,7,10(19)-trien-beta-ol.
Acceptance that Vitamin D Was a Precursor of a Steroid Hormone, 1964-1984
It was clear as of 1964 that the role of vitamin D3 in the general nutrition arena (both human and agricultural) was firmly established. What is surprising, in retrospect, is that almost half a century passed between the specific recognition of the existence of vitamin D in 1921 [11] and the formulation of a theory of the 'mode of action' of vitamin D that was consistent with many if not all of the known facts. This hypothesis states that vitamin D is in reality a steroid and that its mode of action is akin to that of many steroid hormones [27] .
In the intestine, there was a major problem facing biochemists who wished to clarify the detailed mechanism of action of the steroid vitamin D: this was the elucidation of the steps of interaction of the putative steroid hormone with its target tissue and an understanding of how the presence of the steroid hormone initiates the classic physiological response of increased intestinal calcium absorption.
The approach pioneered in the laboratories of Anthony Norman, Egon Kodicek, and Hector DeLuca was to administer small physiological doses of radioactive vitamin D and to trace the appearance of the radioactive label in the target tissue. In the course of these studies, it became apparent not only that there was a specific localization of radioactivity within the target intestinal nuclear and chromatin fraction, but also that this radioactivity was not chemically identical to that of the parent vitamin
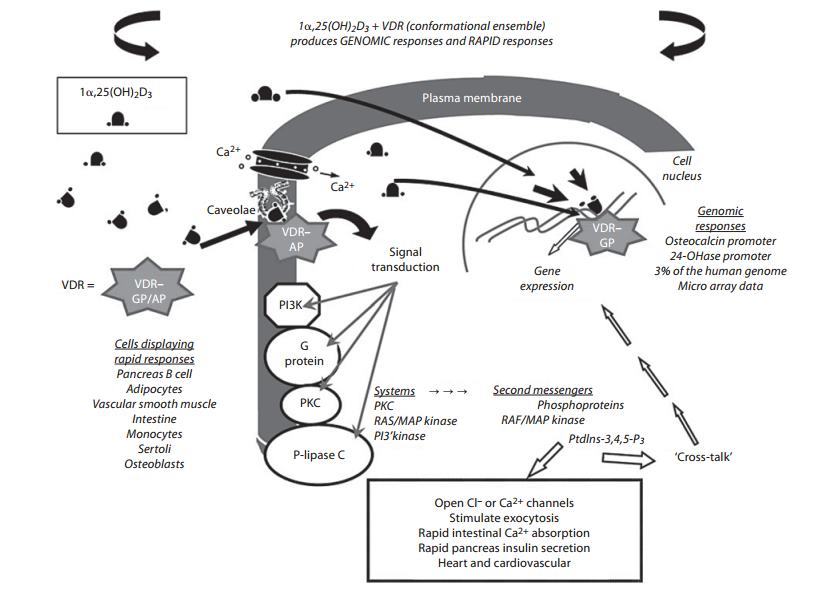
Fig. 2.
1,25(OH)2D3 activation of genomic andnon-genomic (rapid response) cellular signaling. This schematic model illustrates how the conformationally flexible 1,25(OH)2D3 interacts with the VDR in the nucleus to generate genomic responses via regulation of gene transcription, whereas 1a,25(OH)2D3 also binds to VDR associated with caveolae of the plasma membrane to generate non-genomic responses. In the genomic pathway, occupancy of the nuclear VDR by 1a,25(OH)2D3 leads to an up- or downregula-tion of genes subject to hormonal control. The human genome contains about 22,000 genes; of these, approximately 3,000 are regulated by the VDR [41]. Binding of 1a,25(OH)2D3 to caveolae-associated VDR can result in the activation of one or more second-messenger systems, including G-protein-coupled receptors, phosphatidylinositol-3'-kinase (PI3K), or protein kinase C (PKC). A number of possible outcomes exist, including opening of the voltage-gated chloride channels or calcium channels, or generation of the indicated second messengers.
D3. Further studies from the Norman and Kodicek groups demonstrated that this substance, which selectively localized in the target intestine, and its nuclear fraction was chemically different from both vitamin D3 and the intermediate 25-hydroxyvitamin D3 [28, 29]. With the concomitant demonstration that this polar metabolite was —400 X more potent than vitamin D3 in terms of stimulation of the intestinal transport of calcium [28, 30] , the extensive effort necessary to chemically characterize the substance was undertaken. This resulted in simultaneous, yet independent, reports in the first 3 months of 1971 from the three laboratories that the chemical structure of this vitamin D3 metabolite was 1a,25-dihydroxyvitamin D3 [31-33].
Further studies by the Norman laboratory in 19681969 of the specific localization of the tritium radioactivity in the crude nuclear fraction of the intestine revealed that it was also present when purified nuclei were prepared [ 30] or when the subnuclear chromatin fraction was prepared [34, 35].
Fig. 3.
Contribution of vitamin D to good health. The three columns on the right side, respectively, indicate the six physiological systems that the essential nutrient vitamin D3 supports by its metabolism to 25(OH)D3 and 1 a,25(OH)2D3; offer examples of biological responses generated by 1a,25(OH)2D3 in the sixphysiolog-ical systems, and identify for each system some of the disease states that are associated with an inadequate vitamin D nutritional status. More detail is provided by Norman and Bouillon [40] and Bouillon et al. [41]. The Food and Nutrition Board of the Institute of Medicine in 1997 defined serum 25(OH)D levels as a surrogate marker for describing vitamin D nutritional status. Serum 25(OH)D levels entered in the table in the lower left corner describe the 'total' concentration of 25(OH)D, i.e. the sum of the concentration of 25(OH)D3 and 25(OH)D2 present in a serum sample. The use of total serum levels of 25(OH)D as a marker for vitamin D nutritional status is justified by the following three points: (1) there is no clinical assay for the parent vitamin D; (2) the metabolism of vitamin D3 into 25(OH)D3 by the liver vitamin D-25-hydroxylase is not regulated and thus the serum concentration of 25(OH)D3 is believed to be an accurate 'reporter' of both cutaneous ultraviolet-stimulated synthesis and dietary intake of vitamin D3, and (3) the plasma levels of 25(OH)D correlate with manyclinical disease states [50, 51].
This permitted the first isolation and characterization of a specific binding protein (receptor) for 1,25(OH)2D3 [36]; this protein is now known as the vitamin D receptor (VDR).
A clinical application of 1,25(OH)2D3 immediatelybe-came apparent in 1971 in the field of chronic kidney disease which is characterized by the presence of the bone disease of renal osteodystrophy. The onset of the bone disease is caused by damage to the kidney's proximal tubule which inactivates the 25(OH)D-1a-hydroxylase enzyme which converts 25(OH)D3 into the steroid hormone 1,25(OH)2 D3. Thus, the patient will become deficient in 1,25(OH)2 D3. The first treatment of patients with renal osteodystrophy was carried out by oral dosing of 1,25(OH)2D3 by the laboratories of Jack Coburn and Anthony Norman [37, 38] .
Expansion of the Vitamin D Endocrine System, 1987-2012
A general mechanism of the steroid hormone action of 1a,25(OH)2D3 in collaboration with its cognate VDR describing both genomic and non-genomic signal trans-duction responses is presented in figure 2. These more recent developments occurred over the interval of 19872012 [39].
VDR, the receptor for the steroid hormone 1a,25(OH)2D3, is now known to be widely distributed in over 40 tissues, applying the endocrine paradigm that if a cell expresses the receptor for a hormonal ligand, then that cell will be empowered to produce ligand VDR-de-pendent biological responses. Collectively, this suggests that these cells can produce a wide array of biological responses beyond intestinal calcium absorption and the prevention of rickets and osteomalacia [40] .
Figure 3 summarizes all the contributions of the parent vitamin D3 to good health after its metabolism to 1,25(OH)2D3 and binding to its widely distributed VDR
[40, 41]. Thus, the vitamin D endocrine system extends far beyond the classical calcium homeostasis system. It now is clearly involved in the pancreas cell and secretion of insulin and type 2 diabetes [42], the heart and cardiovascular system [43]. the immune system (both the innate and adaptive component) [44], muscle (and muscle strength) [45], and likely the brain. More detailed information is available in Norman and Bouillon [40] and Bouillon et al. [41] .
Vitamin D and Nobel Prizes - Some Near Misses
Nobel Prizes have been awarded to two highly distinguished scientists each of whom made significant contributions in the field of vitamin D research after receiving their prestigious awards.
Prof. Adolf Windaus received the Nobel Prize in Chemistry in 1928 for 'his work on steroids and their relation to vitamins'. In 1919, his laboratory transformed cholesterol into cholanic acid (steroids comprising the bile acids, generally in conjugated form), and in 1926, he proved that the precursor of vitamin D is present in samples of cholesterol and is converted into vitamin D by exposure to sunlight. However, it was only after Windaus received the Nobel Prize that he discovered and synthetically prepared vitamin D3 [22]; it is the naturally occurring form of vitamin D that is most important in preventing the bone disease rickets.
Dorothy Crowfoot Hodgkin was awarded the Nobel Prize in Chemistry in 1964 'for her determinations by X-ray techniques of the structures of important biochemical substances'. She is regarded as one of the pioneer scientists in the field of X-ray crystallography studies of bio-molecules. Her Nobel Award cited her most influential discoveries were 'for the x-ray structures of penicillin and vitamin B12'. It is important to note that Dorothy Crowfoot's PhD dissertation used the pioneering technique of small molecule X-ray diffraction to define unequivocally the chemical structure of vitamin D3 [25, 26] .
Prof. Robert Koch (1843-1910) received his Nobel Prize in Physiology or Medicine in 1905 for his discovery of Mycobacterium tuberculosis, the bacterium that causes tuberculosis. The concept of use of a sanatorium for treatment of patients with tuberculosis was included by Prof. Koch in his 1905 Nobel Lecture [46]. The standard medical therapy for decades to come was based on rest in a sanatorium at a mountain elevation where ultraviolet light was amply prevalent. In 2012, tuberculosis is second only to HIV/AIDS as the greatest killer worldwide due to a single infectious agent. In 2010, 8.8 million people became ill with tuberculosis and 1.4 million died from it. In 2006, new data supported a link between vitamin D deficiency [as determined by the blood levels of 25(OH)D] and tuberculosis and macrophage toll-like receptors stimulated by the VDR + 1a,25(OH)2D3 activation of the innate immunity system [47].
References
1 Whistler D: De morbo puerili Anglorum, quem patrio idiomate indigenae vocant The Rickets. Leiden, ex. Off. W.C. Boxii, 1645.
2 Glisson F: A treatise of the rickets being a disease common to children. Cambridge, Cambridge University, 1660.
3 Trousseau A, Lasegue C: Du rachitisme et de l'osteomalacie compares. Arch Gen Med 1849;19:257.
4 Pommer G: Untersuchungen iiber Osteoma-lacie und Rachitis. Leipzig, F.C.W. Vogel, 1885. Vitamin D3 and Its Steroid Hormone 1a,25(OH)2D3
5 Schiitte D: Beobachtungen iiber den Nutzen des Berger Leberthrans (Oleum jecoris As-seli, von Gadus asellus L.). Arch med Erfahr 1824; 2: 79-92.
6 Palm TA: The geographical distribution and etiology of rickets. Practitioner 1890;45:270-279.
7 Hopkins FG: The analyst and the medical man. Analyst 1906;31:385-397.
8 Hess AF: Rickets including osteomalacia and tetany. Philadelphia, Lea and Febiger, 1929.
9 Mellanby E: An experimental investigation on rickets. Lancet 1919;1:407-412.
10 Mellanby E: The part played by an 'accessory factor' in the production of experimental rickets. J Physiol 1918-1919;52:xi-xii, liii-liv.
11 Mellanby E: Experimental rickets. Medical Research Council Special Report Series No. 61. London, His Majesty's Stationery Office, 1921.
12 Platt BS: Sir Edward Mellanby, 1884-1955: the man, research worker, and statesman. Annu Rev Biochem 1956;25:1-28.
13 Huldschinsky K: Heilung von Rachitis durch kiinstliche Hohensonne. Dtsch med Wschr 1919; 45: 712-713.
14 McCollum EV, Simmonds N, Becker JE, Shipley PG: Studies on experimental rickets. XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem 1922; 53: 293312.
15 Goldblatt H: A study of the relation of the quantity of fat-soluble organic factor in the diet to the degree of calcification of the bones and the development of experimental rickets in rats. Biochem J 1923; 17: 298-326.
16 Goldblatt H, Soames KN: A study of rats on a normal diet irradiated daily by the mercury vapor quartz lamp or kept in darkness. Bio-chem J 1923;17:294-297.
17 Steenbock H, Black A: Fat-soluble vitamins. XVII. The induction of growth-promoting and calcifying properties in a ration by exposure to ultra-violet light. J Biol Chem 1924; 61: 405-422.
18 Steenbock H, Black A, Nelson MT, Hoppert
CA, Riising BM: Fat- soluble vitamins. XXIII. The induction of growth-promoting and calcifying properties in fats and their unsapon-ifiable constituents by exposure to light. Methods Enzymol 1925; 64:263-298.
19 Hess AF, Weinstock M: The antirachitic value of irradiated cholesterol and phytosterol. III. Evidence of chemical change as shown by absorption spectra. Methods Enzymol 1925; 64:193-201.
20 Hess AF, Weinstock M: The antirachitic value of irradiated cholesterol and phytosterol. II. Further evidence of change in biological activity. Methods Enzymol 1925;64:181-191.
21 Windaus A, Linsert O, Luttringhaus A, Weidlinch G: Uber das krystallisierte Vitamin D2. Justus Liebigs Ann Chem 1932; 492:226-231.
22 Angus TC, Askew FA, Bourdillon RB, Bruce HM, Callow R, Fischmann C, Philpot L, Webster TA: A crystalline antirachitic substance. Proc Royal Soc Ser B 1931; 108: 340359.
23 Windaus A, Schenck F, von Werder F: Uber das antirachitisch wirksame Bestrahlungs-produkt aus 7-Dehydrocholesterin. Hoppe-Seylers Ztschr physiol Chem 1936;241:100-103.
24 Brockmann H: Die Isolierung des antirachi-tischen Vitamins aus Thunfischleberol. Hoppe-Seylers Ztschr physiol Chem 1936; 241: 104-115.
25 Crowfoot D, Dunitz JD: Structure of calciferol. Nature 1948; 162: 608-609.
26 Crowfoot-Hodgkin DS, Rummer BM, Du-nitz JD, Trueblood KN: The crystal structure of a calciferol derivative. J Chem Soc 1963; 4945-4956.
27 Norman AW: The mode of action of vitamin D. Biol Rev 1968;43:97-137.
28 Myrtle JF, Haussler MR, Norman AW: Evidence for the biologically active form of cho-lecalciferol in the intestine (Note: This paper was subsequently selected as a 'Nutrition Classic' by Nutrition Reviews 1991;49:302-305). J Biol Chem 1970; 245:1190-1196.
29 Kodicek E, Lawson DEM, Wilson PW: Biological activity of a polar metabolite of vitamin D. Nature 1970; 228: 763.
30 Haussler MR, Myrtle JF, Norman AW: The association of a metabolite of vitamin D3 with intestinal mucosa chromatin, in vivo. J Biol Chem 1968; 243:4055-4064.
31 Norman AW, Myrtle JF, Midgett RJ, Nowicki HG, Williams V, Popjak G: 1,25-Dihydroxy-cholecalciferol: identification of the proposed active form of vitamin D3 in the intestine. Science 1971; 173: 51-54.
32 Lawson DEM, Fraser DR, Kodicek E, Morris HR, Williams DH: Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature 1971; 230: 228-230.
33 Holick MF, Schnoes HK, DeLuca HF: Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in
the intestine. Proc Natl Acad Sci USA 1971; 68:803-804.
34 Haussler MR, Norman AW: Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci USA 1969; 62: 155-162.
35 Haussler MR, Norman AW: Chromosomal receptor for a vitamin D metabolite: a nutrition classic. Nutr Rev 1985;43:181-183.
36 Hunziker W, Walters MR, Bishop JE, Norman AW: Studies on the mode of action of calciferol XXXVIII: effect of vitamin D status on the equilibrium between occupied and unoccupied 1,25-dihydroxyvitamin D receptors. J Clin Invest 1982; 69: 826-834.
37 Brickman AS, Coburn JW, Norman AW: Action of 1,25-dihydroxycholecalciferol, a potent, kidney-produced metabolite of vitamin D3, in uremic man. N Engl J Med 1972; 287: 891-895.
38 Brickman AS, Coburn JW, Massry SG, Norman AW: 1,25-Dihydroxyvitamin D3 in normal man and patients with renal failure. Ann Intern Med 1974;80:161-168.
39 Haussler MR, Jurutka PW, Mizwicki M, Norman AW: Vitamin D receptor (VDR)-mediated actions of 1ct,25(OH)2 vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab 2011; 25: 543-559.
40 Norman AW, Bouillon R: Vitamin D nutritional policy needs a vision for the future. Exp Biol Med 2010; 235: 1034-1045.
41 Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M: Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008; 29: 726-776.
42 Norman AW, Frankel BJ, Heldt AM, Grodsky GM: Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980; 209:823-825.
43 Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC: Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 2004;288:E125-E132.
44 Schauber J, Dorschner RA, Coda AB, Bu-chau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U,
Bikle DD, Modlin RL, Gallo RL: Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-de-pendent mechanism. J Clin Invest 2007; 117: 803-811.
45 Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T: Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myo-regulatory transcription factors. Endocrinology 2003; 144: 5138-5144.
46 Nobelstiftelsen: Les Prix Nobel en 1905. Stockholm, Imprimerie Royale, P.A. Nor-stedt & Soner, 1906.
47 Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL: Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770-1773.
48 Armas LAG, Hollis BW, Heaney RP: Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004; 89: 5387-5391.
49 Heaney RP, Recker RR, Grote J, Horst RL, Armas LA: Vitamin D3 is more potent than vitamin D2 in humans. J Clin End Metab 2011;93: 447-452.
50 Wolpowitz D, Gilchrest BA: The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol 2006; 54:301-317.
51 Vieth R: Vitamin D supplementation, 25-hy-droxyvitamin D concentrations, and safety. Am J Clin Nutr 1999; 69: 842-856.
PDF is attached at the bottom of this page
See also VitaminDWiki
History of Vitamin D from 500 million years ago to orthopaedic practice today – 2019
Vitamin D history back to Egyptians and fortification - Aug 2011
Vitamin D pathways and benefits to the body - Dr Norman - 2011
Dr. Norman has organized the Vitamin D Workshop since 1973.
Chart of Vitamin D Benefits by Dr. Norman
Overview Kidney and vitamin D has the following

