Fast responses to Vitamin D – loading dose, nanoemulsion and Calcifediol
Responses: Loading dose <4 days. nanoemulsion <1 day?, Calcifediol xx? days
Long-Term Treatment and Effect of Discontinuation of Calcifediol in Postmenopausal Women with Vitamin D Deficiency: A Randomized Trial
J Bone Miner Res . 2023 Apr;38(4):471-479. doi: 10.1002/jbmr.4776. Epub 2023 Feb 13.
José Luis Pérez-Castrillón 1 2, Antonio Dueñas-Laita 2 3, Carlos Gómez-Alonso 4, Esteban Jódar 5 6, Javier Del Pino-Montes 7 8, Maria Luisa Brandi 9, Fernando Cereto Castro 10, José Manuel Quesada-Gómez 11 12, Laura Gallego López 13, José Manuel Olmos Martínez 14 15 16, María Rosa Alhambra Expósito 11 12, Bernat Galarraga 17 18, Jesús González-Macías 15 16, José Luis Neyro 19 20, Roger Bouillon 21, Gonzalo Hernández-Herrero 22, Nieves Fernández-Hernando 22, Sandra P Chinchilla 22
Response 90 days?
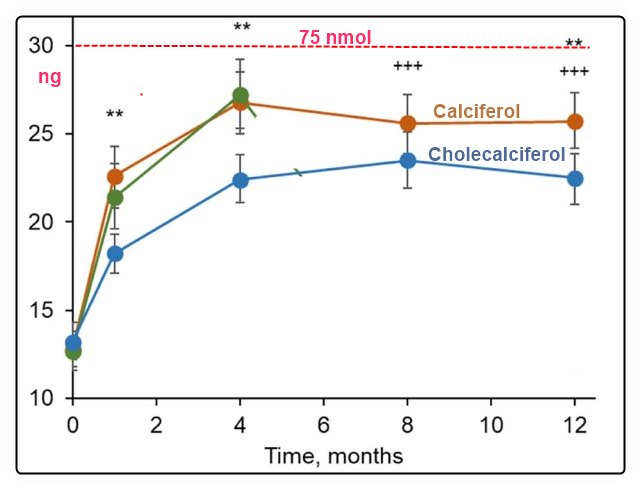
Note: Higher response to D expected if had given 35,000 monthly or 12,500 bi-weekly
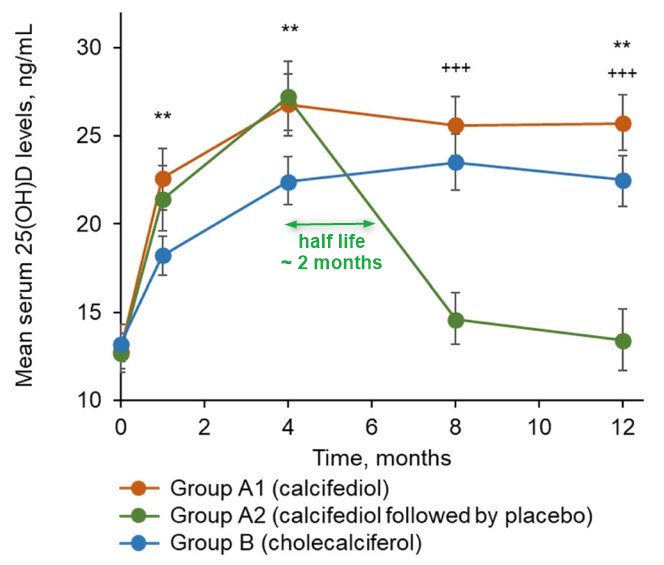
Half-Life
Vitamin D plays a major role in bone health and probably also in multiple extraskeletal acute and chronic diseases. Although supplementation with calcifediol, a vitamin D metabolite, has demonstrated efficacy and safety in short-term clinical trials, its effects after long-term monthly administration have been studied less extensively. This report describes the results of a 1-year, phase III-IV, double-blind, randomized, controlled, parallel, multicenter superiority clinical trial to assess the efficacy and safety of monthly calcifediol 0.266 mg versus cholecalciferol 25,000 IU (0.625 mg) in postmenopausal women with vitamin D deficiency (25(OH)D < 20 ng/mL).
A total of 303 women were randomized and 298 evaluated. Patients were randomized 1:1:1 to
calcifediol 0.266 mg/month for 12 months (Group A1 ),
calcifediol 0.266 mg/month for 4 months followed by placebo for 8 months (Group A2 ), and
cholecalciferol 25,000 IU/month (0.625 mg/month) for 12 months (Group B ).
By month 4, stable 25(OH)D levels were documented with both calcifediol and cholecalciferol (intention-to-treat population): 26.8 ± 8.5 ng/mL (Group A1 ) and 23.1 ± 5.4 ng/mL (Group B ). By month 12, 25(OH)D levels were 23.9 ± 8.0 ng/mL (Group A1 ) and 22.4 ± 5.5 ng/mL (Group B ). When calcifediol treatment was withdrawn in Group A2, 25(OH)D levels decreased to baseline levels (28.5 ± 8.7 ng/mL at month 4 versus 14.4 ± 6.0 ng/mL at month 12). No relevant treatment-related safety issues were reported in any of the groups. The results confirm that long-term treatment with monthly calcifediol in vitamin D-deficient patients is effective and safe. The withdrawal of treatment leads to a pronounced decrease of 25(OH)D levels. Calcifediol presented a faster onset of action compared to monthly cholecalciferol. Long-term treatment produces stable and sustained 25(OH)D concentrations with no associated safety concerns. © 2023 Faes Farma SA. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
📄 Download the PDF from VitaminDWiki
VitaminDWiki – Calcidiol (calcifediol) contains
{include}
