Vitamin D interacts with more than genes (mitochondria, etc)
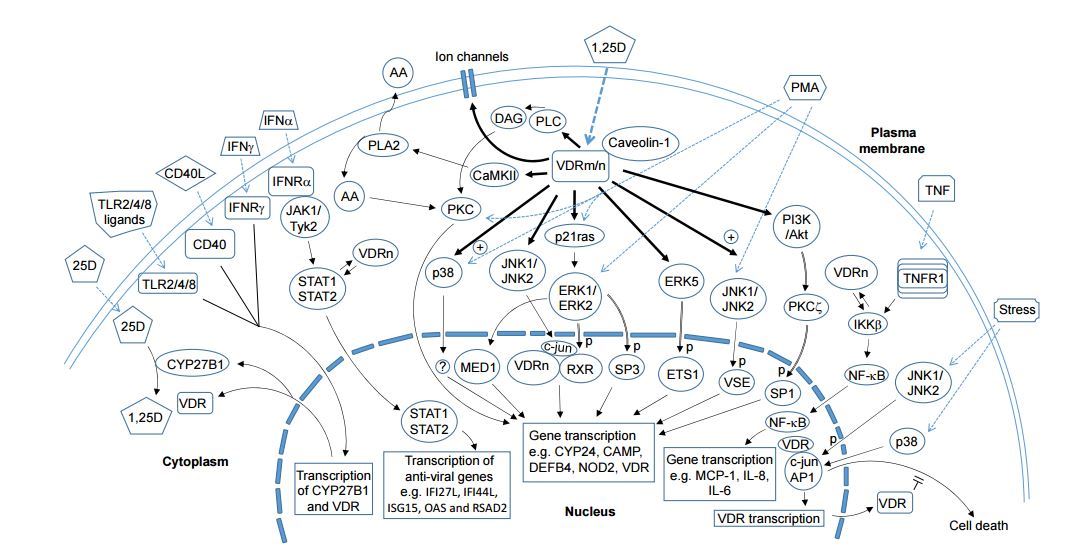
Nongenomic Activities of Vitamin D
Nutrients 2022, 14(23), 5104; https://doi.org/10.3390/nu14235104
Michał A. Żmijewski Department of Histology, Faculty of Medicine, Medical University of Gdańsk, PL-80211 Gdańsk, Poland
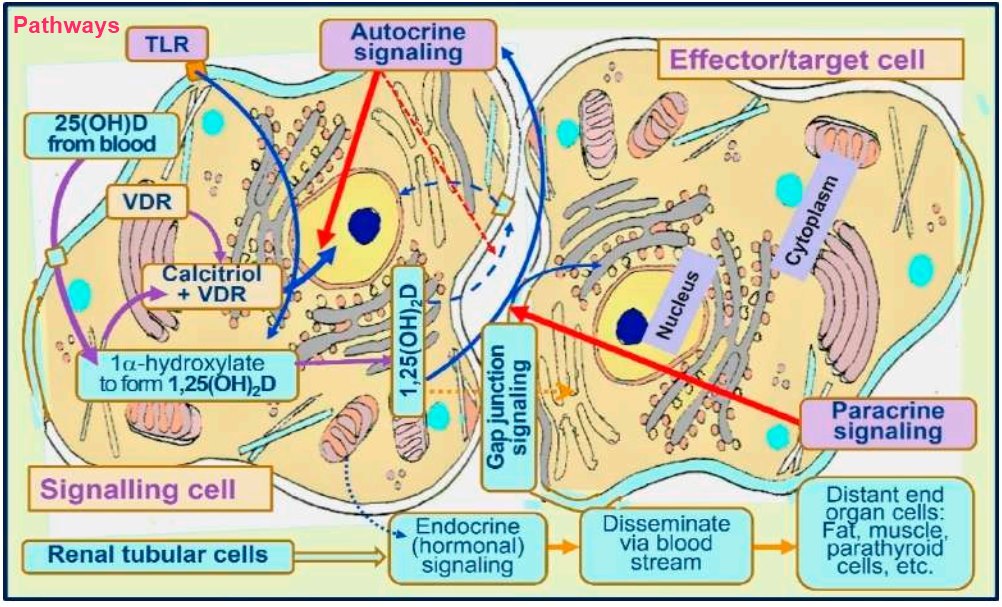
Pathways
PDF Contents

Vitamin D shows a variety of pleiotropic activities which cannot be fully explained by the stimulation of classic pathway- and vitamin D receptor (VDR)-dependent transcriptional modulation. Thus, existence of rapid and nongenomic responses to vitamin D was suggested. An active form of vitamin D (calcitriol, 1,25(OH)2D3) is an essential regulator of calcium–phosphate homeostasis, and this process is tightly regulated by VDR genomic activity. However, it seems that early in evolution, the production of secosteroids (vitamin-D-like steroids) and their subsequent photodegradation served as a protective mechanism against ultraviolet radiation and oxidative stress. Consequently, direct cell-protective activities of vitamin D were proven. Furthermore, calcitriol triggers rapid calcium influx through epithelia and its uptake by a variety of cells. Subsequently, protein disulfide-isomerase A3 (PDIA3) was described as a membrane vitamin D receptor responsible for rapid nongenomic responses.
Vitamin D was also found to stimulate a release of secondary massagers and modulate several intracellular processes—including cell cycle, proliferation, or immune responses—through wingless (WNT), sonic hedgehog (SSH), STAT1-3, or NF-kappaB pathways.
Megalin and its coreceptor, cubilin, facilitate the import of vitamin D complex with vitamin-D-binding protein (DBP), and its involvement in rapid membrane responses was suggested.
Vitamin D also directly and indirectly influences mitochondrial function , including
fusion–fission,
energy production,
mitochondrial membrane potential,
activity of ion channels, and
apoptosis.
Although mechanisms of the nongenomic responses to vitamin D are still not fully understood, in this review, their impact on physiology, pathology, and potential clinical applications will be discussed.
📄 Download the PDF from VitaminDWiki
VitaminDWiki - Vitamin D actions which are faster than gene interactions – March 2016
The Non-Genomic Actions of Vitamin D
References
Carlberg, C. Vitamin D in the Context of Evolution . Nutrients 2022,14, 3018. [CrossRef]
Hanel, A.; Carlberg, C. Vitamin D and evolution : Pharmacologic implications. Biochem. Pharmacol. 2020,173,113595. [CrossRef]
Holick, M.F. Vitamin D: Evolutionary, physiological and health perspectives. Curr. Drug Targets 2011, 12, 4-18. [CrossRef] [PubMed]
Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm.-Endocrinol. 2013, 5, 51-108. [CrossRef] [PubMed]
Holick, M.F.; Frommer, J.E.; McNeill, S.C.; Richtand, N.M.; Henley, J.W.; Potts, J.T., Jr. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem. Biophys. Res. Commun. 1977, 76,107-114. [CrossRef] [PubMed]
Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action —Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020,16,234-252. [CrossRef]
Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012,13, 3-19. [CrossRef]
Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019,10, 317. [CrossRef]
Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2019,10, 910. [CrossRef]
Holick, M.F.; Clark, M.B. The photobiogenesis and metabolism of vitamin D. Fed. Proc. 1978, 37, 2567-2574.
Fleet, J.C. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell. Endocrinol. 2017, 453, 36-45. [CrossRef] [PubMed]
Pludowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokol, D.; Czech-Kowalska, J.; Debski, R.; Decsi, T.; Dobrzanska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—Recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319-327. [CrossRef] [PubMed]
Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125-135. [CrossRef] [PubMed]
Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018,177, 266-269. [CrossRef]
Amon, U.; Baier, L.; Yaguboglu, R.; Ennis, M.; Holick, M.F.; Amon, J. Serum 25-hydroxyvitamin D levels in patients with skin diseases including psoriasis, infections, and atopic dermatitis. Derm.-Endocrinol. 2018,10, e1442159. [CrossRef]
Takeyama, K.; Kitanaka, S.; Sato, T.; Kobori, M.; Yanagisawa, J.; Kato, S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science 1997, 277,1827-1830. [CrossRef]
Holick, M.F.; Uskokovic, M.; Henley, J.W.; MacLaughlin, J.; Holick, S.A.; Potts, J.T. The photoproduction of 1 alpha,25- dihydroxyvitamin D3 in skin: An approach to the therapy of vitamin-D-resistant syndromes. N. Engl. J. Med. 1980, 303, 349-354. [CrossRef]
Webb, A.R.; DeCosta, B.R.; Holick, M.F. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 1989, 68, 882-887. [CrossRef]
Tuckey, R.C.; Tang, E.K.Y.; Maresse, S.R.; Delaney, D.S. Catalytic properties of 25-hydroxyvitamin D3 3-epimerase in rat and human liver microsomes. Arch. Biochem. Biophys. 2019, 666,16-21. [CrossRef]
Berger, S.E.; Van Rompay, M.I.; Gordon, C.M.; Goodman, E.; Eliasziw, M.; Holick, M.F.; Sacheck, J.M. Investigation of the C-3-epi-25(OH)D. Appl. Physiol. Nutr. Metab. 2018, 43, 259-265. [CrossRef]
Kmiec, P.; Minkiewicz, I.; Sworczak, K.; Zmijewski, M.A.; Kowalski, K. Vitamin D status including 3-epi-25(OH)D3 among adult patients with thyroid disorders during summer months. Endokrynol. Pol. 2018, 69, 653-660. [CrossRef] [PubMed]
Jones, G.; Kaufmann, M. Diagnostic Aspects of Vitamin D : Clinical Utility of Vitamin D Metabolite Profiling. JBMR Plus 2021, 5, e10581. [CrossRef] [PubMed]
Chen, J.; Tang, Z.; Slominski, A.T.; Li, W.; Zmijewski, M.A.; Liu, Y. Vitamin D and its analogs as anticancer and anti-inflammatory agents. Eur. J. Med. Chem. 2020, 207,112738. [CrossRef] [PubMed]
Slominski, A.T.; Brozyna, A.A.; Skobowiat, C.; Zmijewski, M.A.; Kim, T.K.; Janjetovic, Z.; Oak, A.S.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J. Steroid Biochem. Mol. Biol. 2018,177,159-170. [CrossRef] [PubMed]
Tuckey, R.C.; Li, W.; Zjawiony, J.K.; Zmijewski, M.A.; Nguyen, M.N.; Sweatman, T.; Miller, D.; Slominski, A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008, 275, 2585-2596. [CrossRef]
Slominski, A.T.; Semak, I.; Zmijewski, M.A.; Sweatman, T.; Janjetovic, Z.; Wortsman, J.; Tuckey, R.C. Sequential metabolism of provitamin D3 (7-dehydrocholesterol) to 5,7-diene products in the adrenal gland. J. Investig. Dermatol. 2007,127, 443.
Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1a,25(OH)2 vitamin D3: Genomic and non-genomic mechanisms. BestPract. Res. Clin. Endocrinol. Metab. 2011,25,543-559. [CrossRef]
Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Brozyna, A.A.; Zmijewski, M.A.; Xu, H.; Sutter, T.R.; Tuckey, R.C.; Jetten, A.M.; Crossman, D.K. Differential and Overlapping Effects of 20,23(OH)2D3 and 1,25(OH)2D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)2D3. Int. J. Mol. Sci. 2018,19, 3072. [CrossRef]
Carlberg, C. Vitamin D and Its Target Genes . Nutrients 2022,14,1354. [CrossRef]
Hanel, A.; Veldhuizen, C.; Carlberg, C. Gene-Regulatory Potential of 25-Hydroxyvitamin D. Front. Nutr. 2022, 9, 910601. [CrossRef]
Hanel, A.; Bendik, I.; Carlberg, C. Transcriptome-Wide Profile of 25-Hydroxyvitamin D. Nutrients 2021,13, 4100. [CrossRef]
Ellfolk, M.; Norlin, M.; Gyllensten, K.; Wikvall, K. Regulation of human vitamin D(3) 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol. Pharmacol. 2009, 75,1392-1399. [CrossRef]
Susa, T.; Iizuka, M.; Okinaga, H.; Tamamori-Adachi, M.; Okazaki, T. Without 1 a-hydroxylation, the gene expression profile of 25(OH)D. Sci. Rep. 2018, 8, 9024. [CrossRef] [PubMed]
Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORalpha and RORgamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775-2789. [CrossRef] [PubMed]
Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as "biased" agonists on VDR and inverse agonists on RORa and RORy. J. Steroid Biochem. Mol. Biol. 2017,173, 42-56. [CrossRef] [PubMed]
Warwick, T.; Schulz, M.H.; Günther, S.; Gilsbach, R.; Neme, A.; Carlberg, C.; Brandes, R.P.; Seuter, S. A hierarchical regulatory network analysis of the vitamin D induced transcriptome reveals novel regulators and complete VDR dependency in monocytes. Sci. Rep. 2021,11,6518. [CrossRef] [PubMed]
Gallardo Martin, E.; Cousillas Castiñeiras, A. Vitamin D modulation and microRNAs in gastric cancer: Prognostic and therapeutic role. Transl. Cancer Res. 2021,10,3111-3127. [CrossRef]
Shahrzad, M.K.; Gharehgozlou, R.; Fadaei, S.; Hajian, P.; Mirzaei, H.R. Vitamin D and Non-coding RNAs: New Insights into the Regulation of Breast Cancer. Curr. Mol. Med. 2021, 21,194-210. [CrossRef]
Charoenngam, N.; Ayoub, D.; Holick, M.F. Nutritional rickets and vitamin D deficiency: Consequences and strategies for treatment and prevention. Expert Rev. Endocrinol. Metab. 2022,17, 351-364. [CrossRef]
Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016, 4,16041. [CrossRef]
Wierzbicka, J.; Piotrowska, A.; Zmijewski, M.A. The renaissance of vitamin D. Acta Biochim. Pol. 2014, 61, 679-686. [CrossRef] [PubMed]
Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017,18,153-165. [CrossRef] [PubMed]
Yamamoto, E.A.; J0rgensen, T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2019,10, 3141. [CrossRef] [PubMed]
Holmes, E.A.; Rodney Harris, R.M.; Lucas, R.M. Low Sun Exposure and Vitamin D Deficiency as Risk Factors for Inflammatory Bowel Disease, With a Focus on Childhood Onset. Photochem. Photobiol. 2019, 95,105-118. [CrossRef]
Mak, A. The Impact of Vitamin D on the Immunopathophysiology, Disease Activity, and Extra-Musculoskeletal Manifestations of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2018,19, 2355. [CrossRef]
Medrano, M.; Carrillo-Cruz, E.; Montero, I.; Perez-Simon, J.A. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. Int. J. Mol. Sci. 2018,19, 2663. [CrossRef]
Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the Immune System: Genomic and Non-Genomic Actions. Mini Rev. Med. Chem. 2015,15, 953-963. [CrossRef]
Gruber-Bzura, B.M. Vitamin D and Influenza-Prevention or Therapy? Int. J. Mol. Sci. 2018,19, 2419. [CrossRef]
Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D and Its Potential Benefit for the COVID-19 Pandemic. Endocr. Pract. 2021, 27, 484-493. [CrossRef]
Giustina, A.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Lazaretti-Castro, M.; Lips, P.; Marcocci, C.; Bilezikian, J.P. Vitamin D in the older population: A consensus statement. Endocrine 2022. [CrossRef]
Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. itamin D and the brain : Genomic and non-genomic actions. Mol. Cell. Endocrinol. 2017, 453,131-143. [CrossRef] [PubMed]
Eyles, D.W. Vitamin D: Brain and Behavior. JBMR Plus 2021, 5, e10419. [CrossRef] [PubMed]
Owczarczyk-Saczonek, A.; Purzycka-Bohdan, D.; Nedoszytko, B.; Reich, A.; Szczerkowska-Dobosz, A.; Bartosiñska, J.; Batycka- Baran, A.; Czajkowski, R.; Dobrucki, I.T.; Dobrucki, L.W.; et al. Pathogenesis of psoriasis in the "omic" era. Part III. Metabolic disorders, metabolomics, nutrigenomics in psoriasis. Postepy Dermatol. Alergol. 2020, 37, 452-467. [CrossRef] [PubMed]
Carlberg, C.; Velleuer, E. Vitamin D and the risk for cancer : A molecular analysis. Biochem. Pharmacol. 2022, 196, 114735. [CrossRef]
Holick, M.F. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? Adv. Exp. Med. Biol. 2020, 1268,19-36. [CrossRef]
Ferrer-Mayorga, G.; Larriba, M.J.; Crespo, P.; Muñoz, A. Mechanisms of action of vitamin D in colon cancer. J. Steroid Biochem. Mol. Biol. 2019,185,1-6. [CrossRef]
Reichrath, J.; Saternus, R.; Vogt, T. Endocrine actions of vitamin D in skin: Relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol. Cell. Endocrinol. 2017, 453, 96-102. [CrossRef]
De Smedt, J.; Van Kelst, S.; Boecxstaens, V.; Stas, M.; Bogaerts, K.; Vanderschueren, D.; Aura, C.; Vandenberghe, K.; Lambrechts, D.; Wolter, P.; et al. Vitamin D supplementation in cutaneous malignant melanoma outcome (ViDMe): A randomized controlled trial. BMC Cancer 2017,17, 562. [CrossRef]
Piotrowska, A.; Wierzbicka, J.; Zmijewski, M.A. Vitamin D in the skin physiology and pathology. Acta Biochim. Pol. 2016, 63, 17-29. [CrossRef]
Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients. 2022, 14,1448. [CrossRef]
Moretti, R.; Morelli, M.E.; Caruso, P. Vitamin D in Neurological Diseases: A Rationale for a Pathogenic Impact. Int. J. Mol. Sci. 2018,19, 2245. [CrossRef] [PubMed]
Dziedzic, E.A.; Grant, W.B.; Sowinska, I.; Dabrowski, M.; Jankowski, P. Small Differences in Vitamin D Levels between Male Cardiac Patients in Different Stages of Coronary Artery Disease. J. Clin. Med. 2022,11, 779. [CrossRef] [PubMed]
Boucher, B.J.; Grant, W.B. Difficulties in designing randomised controlled trials of vitamin D supplementation for reducing acute cardiovascular events and in the analysis of their outcomes. Int. J. Cardiol. Heart Vasc. 2020,29,100564. [CrossRef]
Duffy, M.J.; Murray, A.; Synnott, N.C.; O'Donovan, N.; Crown, J. Vitamin D analogues: Potential use in cancer treatment. Crit. Rev. Oncol. Hematol. 2017,112,190-197. [CrossRef]
Tabatabaeizadeh, S.A.; Avan, A.; Bahrami, A.; Khodashenas, E.; Esmaeili, H.; Ferns, G.A.; Abdizadeh, M.F.; Ghayour-Mobarhan, M. High Dose Supplementation of Vitamin D Affects Measures of Systemic Inflammation: Reductions in High Sensitivity C-Reactive Protein Level and Neutrophil to Lymphocyte Ratio (NLR) Distribution. J. Cell. Biochem. 2017, 118, 4317-4322. [CrossRef]
Rosen, Y.; Daich, J.; Soliman, I.; Brathwaite, E.; Shoenfeld, Y. Vitamin D and autoimmunity. Scand. J. Rheumatol. 2016, 45,439-447. [CrossRef] [PubMed]
Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945-1960. [CrossRef]
Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Derm.-Endocrinol. 2013, 5,222-234. [CrossRef]
Suarez-Varela, M.M.; Ugar, N.; Peraita-Costa, I.; Huertas, M.F.; Soriano, J.M.; Llopis-Morales, A.; Grant, W.B. Vitamin D-Related Risk Factors for Maternal Morbidity during Pregnancy: A Systematic Review. Nutrients 2022,14, 3166. [CrossRef]
Pludowski, P.; Ducki, C.; Konstantynowicz, J.; Jaworski, M. Vitamin D status in Poland. Pol. Arch. Med. Wewn. 2016,126, 530-539. [CrossRef]
Kmiec, P.; Zmijewski, M.; Waszak, P.; Sworczak, K.; Lizakowska-Kmiec, M. Vitamin D deficiency during winter months among an adult, predominantly urban, population in Northern Poland. Endokrynol. Pol. 2014, 65,105-113. [CrossRef] [PubMed]
Kmiec, P.; Zmijewski, M.; Lizakowska-Kmiec, M.; Sworczak, K. Widespread vitamin D deficiency among adults from northern Poland (54° N) after months of low and high natural UVB radiation. Endokrynol. Pol. 2015, 66, 30-38. [CrossRef] [PubMed]
Thiebaut, C.; Vlaeminck-Guillem, V.; Tredan, O.; Poulard, C.; Le Romancer, M. Non-genomic signaling of steroid receptors in cancer. Mol. Cell. Endocrinol. 2021, 538,111453. [CrossRef]
Khanal, R.C.; Nemere, I. The ERp57/GRp58/1,25D3-MARRS receptor: Multiple functional roles in diverse cell systems. Curr. Med. Chem. 2007,14,1087-1093. [CrossRef]
Civitelli, R.; Kim, Y.S.; Gunsten, S.L.; Fujimori, A.; Huskey, M.; Avioli, L.V.; Hruska, K.A. Nongenomic activation of the calcium message system by vitamin D metabolites in osteoblast-like cells. Endocrinology 1990,127, 2253-2262. [CrossRef] [PubMed]
Selles, J.; Boland, R. Evidence on the participation of the 3,,5,-cyclic AMP pathway in the non-genomic action of 1,25-dihydroxy- vitamin D3 in cardiac muscle. Mol. Cell. Endocrinol. 1991, 82, 229-235. [CrossRef] [PubMed]
Fleet, J.C.; Schoch, R.D. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit. Rev. Clin. Lab. Sci. 2010, 47,181-195. [CrossRef]
Sterling, T.M.; Nemere, 1.1,25-dihydroxyvitamin D3 stimulates vesicular transport within 5 s in polarized intestinal epithelial cells. J. Endocrinol. 2005,185, 81-91. [CrossRef]
Tunsophon, S.; Nemere, I. Protein kinase C isotypes in signal transduction for the 1,25D3-MARRS receptor (ERp57/PDIA3) in steroid hormone-stimulated phosphate uptake. Steroids 2010, 75, 307-313. [CrossRef]
Nemere, I.; Farach-Carson, M.C.; Rohe, B.; Sterling, T.M.; Norman, A.W.; Boyan, B.D.; Safford, S.E. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc. Natl. Acad. Sci. USA 2004,101, 7392-7397. [CrossRef]
Boyan, B.D.; Sylvia, V.L.; McKinney, N.; Schwartz, Z. Membrane actions of vitamin D metabolites 1alpha,25(OH)2D3 and 24R,25(OH)2D3 are retained in growth plate cartilage cells from vitamin D receptor knockout mice. J. Cell. Biochem. 2003, 90, 1207-1223. [CrossRef]
Bikle, D.D. The Free Hormone Hypothesis: When, Why, and How to Measure the Free Hormone Levels to Assess Vitamin D, Thyroid, Sex Hormone, and Cortisol Status. JBMR Plus 2021, 5, e10418. [CrossRef]
Willnow, T.E.; Nykjaer, A. Cellular uptake of steroid carrier proteins—Mechanisms and implications. Mol. Cell. Endocrinol. 2010, 316, 93-102. [CrossRef] [PubMed]
Safadi, F.F.; Thornton, P; Magiera, H.; Hollis, B.W.; Gentile, M.; Haddad, J.G.; Liebhaber, S.A.; Cooke, N.E. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J. Clin. Investig. 1999,103, 239-251. [CrossRef]
Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; de la Chapelle, A.; Moestrup, S.K.; et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc. Natl. Acad. Sci. USA 2001, 98,13895-13900. [CrossRef] [PubMed]
Leheste, J.R.; Rolinski, B.; Vorum, H.; Hilpert, J.; Nykjaer, A.; Jacobsen, C.; Aucouturier, P; Moskaug, J.O.; Otto, A.; Christensen, E.I.; et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 1999,155, 1361-1370. [CrossRef] [PubMed]
Leheste, J.R.; Meisen, F.; Wellner, M.; Jansen, P.; Schlichting, U.; Renner-Müller, I.; Andreassen, T.T.; Wolf, E.; Bachmann, S.; Nykjaer, A.; et al. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003,17, 247-249. [CrossRef] [PubMed]
Abboud, M.; Puglisi, D.A.; Davies, B.N.; Rybchyn, M.; Whitehead, N.P.; Brock, K.E.; Cole, L.; Gordon-Thomson, C.; Fraser, D.R.; Mason, R.S. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology 2013,154, 3022-3030. [CrossRef]
Rowling, M.J.; Kemmis, C.M.; Taffany, D.A.; Welsh, J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J. Nutr. 2006,136, 2754-2759. [CrossRef]
Ternes, S.B.; Rowling, M.J. Vitamin D transport proteins megalin and disabled-2 are expressed in prostate and colon epithelial cells and are induced and activated by all-trans-retinoic acid. Nutr. Cancer 2013, 65, 900-907. [CrossRef]
Negri, A.L. Proximal tubule endocytic apparatus as the specific renal uptake mechanism for vitamin D-binding protein/25- (OH)D3 complex. Nephrology 2006,11, 510-515. [CrossRef] [PubMed]
Gao, Y.; Zhou, S.; Luu, S.; Glowacki, J. Megalin mediates 25-hydroxyvitamin D. FASEB J. 2019,33, 7684-7693. [CrossRef] [PubMed]
Sheikh-Hamad, D.; Holliday, M.; Li, Q. Megalin-Mediated Trafficking of Mitochondrial Intracrines: Relevance to Signaling and Metabolism. J. Cell. Immunol. 2021, 3, 364-369. [PubMed]
Li, Q.; Lei, F.; Tang, Y.; Pan, J.S.; Tong, Q.; Sun, Y.; Sheikh-Hamad, D. Megalin mediates plasma membrane to mitochondria cross-talk and regulates mitochondrial metabolism. Cell. Mol. Life Sci. 2018, 75, 4021-4040. [CrossRef] [PubMed]
Taparia, S.; Fleet, J.C.; Peng, J.B.; Wang, X.D.; Wood, R.J. 1,25-Dihydroxyvitamin D and 25-hydroxyvitamin D—Mediated regulation of TRPV6 (a putative epithelial calcium channel) mRNA expression in Caco-2 cells. Eur. J. Nutr. 2006, 45,196-204. [CrossRef] [PubMed]
Schwartz, N.; Verma, A.; Bivens, C.B.; Schwartz, Z.; Boyan, B.D. Rapid steroid hormone actions via membrane receptors. Biochim. Biophys. Acta 2016,1863, 2289-2298. [CrossRef]
Gerdes, D.; Christ, M.; Haseroth, K.; Notzon, A.; Falkenstein, E.; Wehling, M. Nongenomic Actions of Steroids—From the Laboratory to Clinical Implications. J. Pediatr. Endocrinol. Metab. 2000,13, 853-878. [CrossRef]
Baran, D.T.; Ray, R.; Sorensen, A.M.; Honeyman, T.; Holick, M.F. Binding characteristics of a membrane receptor that recognizes 1a25-dihydroxyvitamin D3 and its epimer, 1ß,25-dihydroxyvitamin D3. J. Cell. Biochem. 1994, 56, 510-517. [CrossRef]
Nemere, I.; Yoshimoto, Y.; Norman, A.W. Calcium Transport in Perfused Duodena from Normal Chicks: Enhancement within Fourteen Minutes of Exposure to 1,25-Dihydroxyvitamin D3*. Endocrinology 1984,115,1476-1483. [CrossRef]
Nemere, I.; Dormanen, M.C.; Hammond, M.W.; Okamura, W.H.; Norman, A.W. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J. Biol. Chem. 1994, 269, 23750-23756. [CrossRef]
Doroudi, M.; Olivares-Navarrete, R.; Boyan, B.D.; Schwartz, Z. A review of 1a,25(OH)2D3 dependent Pdia3 receptor complex components in Wnt5a non-canonical pathway signaling. J. Steroid Biochem. Mol. Biol. 2015,152, 84-88. [CrossRef]
Doroudi, M.; Plaisance, M.C.; Boyan, B.D.; Schwartz, Z. Membrane actions of 1a,25(OH)2D3 are mediated by Ca2+/calmodulin- dependent protein kinase II in bone and cartilage cells. J. Steroid Biochem. Mol. Biol. 2015,145, 65-74. [CrossRef] [PubMed]
Nemere, I.; Garbi, N.; Winger, Q. The 1,25D3-MARRS receptor/PDIA3/ERp57 and lifespan. J. Cell. Biochem. 2014,116, 380-385. [CrossRef] [PubMed]
Aureli, C.; Gaucci, E.; Arcangeli, V.; Grillo, C.; Eufemi, M.; Chichiarelli, S. ERp57/PDIA3 binds specific DNA fragments in a melanoma cell line. Gene 2013, 524, 390-395. [CrossRef] [PubMed]
Nemere, I.; Garbi, N.; Hammerling, G.; Hintze, K.J. Role of the 1,25D3-MARRS receptor in the 1,25(OH)2D3-stimulated uptake of calcium and phosphate in intestinal cells. Steroids 2012, 77, 897-902. [CrossRef]
Chen, J.; Olivares-Navarrete, R.; Wang, Y.; Herman, T.R.; Boyan, B.D.; Schwartz, Z. Protein-disulfide Isomerase-associated 3 (Pdia3) Mediates the Membrane Response to 1,25-Dihydroxyvitamin D3 in Osteoblasts. J. Biol. Chem. 2010, 285, 37041-37050. [CrossRef] [PubMed]
Nemere, I.; Garbi, N.; Hämmerling, G.J.; Khanal, R.C. Intestinal Cell Calcium Uptake and the Targeted Knockout of the 1,25D3- MARRS (Membrane-associated, Rapid Response Steroid-binding) Receptor/PDIA3/Erp57. J. Biol. Chem. 2010, 285, 31859-31866. [CrossRef] [PubMed]
Wang, Y.; Chen, J.; Lee, C.S.; Nizkorodov, A.; Riemenschneider, K.; Martin, D.; Hyzy, S.; Schwartz, Z.; Boyan, B.D. Disruption of Pdia3 gene results in bone abnormality and affects 1 a,25-dihydroxy-vitamin D3-induced rapid activation of PKC. J. Steroid Biochem. Mol. Biol. 2010,121, 257-260. [CrossRef]
Nemere, I.; Safford, S.E.; Rohe, B.; DeSouza, M.M.; Farach-Carson, M.C. Identification and characterization of 1,25D3-membrane- associated rapid response, steroid (1,25D3-MARRS) binding protein. J. Steroid Biochem. Mol. Biol. 2004, 89-90, 281-285. [CrossRef]
Garbi, N.; Tanaka, S.; Momburg, F.; Hämmerling, G.J. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat. Immunol. 2005, 7, 93-102. [CrossRef]
Doroudi, M.; Chen, J.; Boyan, B.D.; Schwartz, Z. New insights on membrane mediated effects of 1 a,25-dihydroxy vitamin D3 signaling in the musculoskeletal system. Steroids 2014, 81, 81-87. [CrossRef]
Wilkin, A.M.; Harnett, A.; Underschultz, M.; Cragg, C.; Meckling, K.A. Role of the ERp57 protein (1,25D3-MARRS receptor) in murine mammary gland growth and development. Steroids 2018,135, 63-68. [CrossRef] [PubMed]
Hettinghouse, A.; Liu, R.; Liu, C.-J. Multifunctional molecule ERp57: From cancer to neurodegenerative diseases. Pharmacol. Ther. 2018,181, 34-48. [CrossRef] [PubMed]
Wasiewicz, T.; Szyszka, P.; Cichorek, M.; Janjetovic, Z.; Tuckey, R.C.; Slominski, A.T.; Zmijewski, M.A. Antitumor Effects of Vitamin D Analogs on Hamster and Mouse Melanoma Cell Lines in Relation to Melanin Pigmentation. Int. J. Mol. Sci. 2015,16, 6645-6667. [CrossRef]
Gaucci, E.; Raimondo, D.; Grillo, C.; Cervoni, L.; Altieri, F.; Nittari, G.; Eufemi, M.; Chichiarelli, S. Analysis of the interaction of calcitriol with the disulfide isomerase ERp57. Sci. Rep. 2016, 6, 37957. [CrossRef]
Chen, J.; Lobachev, K.S.; Grindel, B.J.; Farach-Carson, M.C.; Hyzy, S.L.; El-Baradie, K.B.; Olivares-Navarrete, R.; Doroudi, M.; Boyan, B.D.; Schwartz, Z. Chaperone properties of pdia3 participate in rapid membrane actions of 1 a,25-dihydroxyvitamin d3. Mol. Endocrinol. 2013, 27,1065-1077. [CrossRef] [PubMed]
Kozlov, G.; Maattanen, P.; Schrag, J.D.; Pollock, S.; Cygler, M.; Nagar, B.; Thomas, D.; Gehring, K. Crystal Structure of the bb' Domains of the Protein Disulfide Isomerase ERp57. Structure 2006,14,1331-1339. [CrossRef]
Wang, Y.; Nizkorodov, A.; Riemenschneider, K.; Lee, C.S.D.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Impaired Bone Formation in Pdia3 Deficient Mice. PLoS ONE 2014, 9, e112708. [CrossRef]
Yang, W.S.; Yu, H.; Kim, J.J.; Lee, M.J.; Park, S.K. Vitamin D-induced ectodomain shedding of TNF receptor 1 as a nongenomic action: D3 vs. D2 derivatives. J. Steroid Biochem. Mol. Biol. 2016,155,18-25. [CrossRef]
Li, Y.; Camacho, P. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J. Cell Biol. 2003,164, 35-46. [CrossRef]
Khanal, R.; Nemere, I. Membrane receptors for vitamin D metabolites. Crit. Rev. Eukaryot. Gene Expr. 2007,17, 31-47. [CrossRef] [PubMed]
Sequeira, V.B.; Rybchyn, M.S.; Tongkao-On, W.; Gordon-Thomson, C.; Malloy, P.J.; Nemere, I.; Norman, A.W.; Reeve, V.E.; Halliday, G.M.; Feldman, D.; et al. The role of the vitamin D receptor and ERp57 in photoprotection by 1 a,25-dihydroxyvitamin D3. Mol. Endocrinol. 2012,26, 574-582. [CrossRef] [PubMed]
Egger, A.N.; Rajabi-Estarabadi, A.; Williams, N.M.; Resnik, S.R.; Fox, J.D.; Wong, L.L.; Jozic, I. The importance of caveolins and caveolae to dermatology: Lessons from the caves and beyond. Exp. Dermatol. 2019,29,136-148. [CrossRef] [PubMed]
Doroudi, M.; Schwartz, Z.; Boyan, B.D. Membrane-mediated actions of 1,25-dihydroxy vitamin D3: A review of the roles of phospholipase A2 activating protein and Ca2+/calmodulin-dependent protein kinase II. J. Steroid Biochem. Mol. Biol. 2015,147, 81-84. [CrossRef] [PubMed]
Chichiarelli, S.; Altieri, F.; Paglia, G.; Rubini, E.; Minacori, M.; Eufemi, M. ERp57/PDIA3: New insight. Cell. Mol. Biol. Lett. 2022, 27,1-18. [CrossRef]
Grindel, B.J.; Rohe, B.; Safford, S.E.; Bennett, J.J.; Farach-Carson, M.C. Tumor necrosis factor-a treatment of HepG2 cells mobilizes a cytoplasmic pool of ERp57/1,25D3-MARRS to the nucleus. J. Cell. Biochem. 2011,112, 2606-2615. [CrossRef] [PubMed]
Hu, W.; Zhang, L.; Li, M.X.; Shen, J.; Liu, X.D.; Xiao, Z.G.; Wu, D.L.; Ho, I.H.T.; Wu, J.C.Y.; Cheung, C.K.Y.; et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy 2019,15, 707-725. [CrossRef]
Guo, G.G.; Patel, K.; Kumar, V.; Shah, M.; Fried, V.A.; Etlinger, J.D.; Sehgal, P.B. Association of the Chaperone Glucose-Regulated Protein 58 (GRP58/ER-60/ERp57) with Stat3 in Cytosol and Plasma Membrane Complexes. J. Interf. Cytokine Res. 2002, 22, 555-563. [CrossRef]
Chichiarelli, S.; Gaucci, E.; Ferraro, A.; Grillo, C.; Altieri, F.; Cocchiola, R.; Arcangeli, V.; Turano, C.; Eufemi, M. Role of ERp57 in the signaling and transcriptional activity of STAT3 in a melanoma cell line. Arch. Biochem. Biophys. 2009, 494,178-183. [CrossRef]
Wu, W.; Beilhartz, G.; Roy, Y.; Richard, C.L.; Curtin, M.; Brown, L.; Cadieux, D.; Coppolino, M.; Farach-Carson, M.C.; Nemere, I.; et al. Nuclear translocation of the 1,25D3-MARRS (membrane associated rapid response to steroids) receptor protein and NFkB in differentiating NB4 leukemia cells. Exp. Cell Res. 2010, 316,1101-1108. [CrossRef]
Grillo, C.; D'Ambrosio, C.; Consalvi, V.; Chiaraluce, R.; Scaloni, A.; Maceroni, M.; Eufemi, M.; Altieri, F. DNA-binding Activity of the ERp57 C-terminal Domain Is Related to a Redox-dependent Conformational Change. J. Biol. Chem. 2007,282,10299-10310. [CrossRef]
Doroudi, M.; Olivares-Navarrete, R.; Hyzy, S.L.; Boyan, B.D.; Schwartz, Z. Signaling components of the 1a,25(OH)2D3-dependent Pdia3 receptor complex are required for Wnt5a calcium-dependent signaling. Biochim. Biophys. Acta 2014, 1843, 2365-2375. [CrossRef] [PubMed]
Zhu, L.; Santos, N.C.; Kim, K.H. Disulfide isomerase glucose-regulated protein 58 is required for the nuclear localization and degradation of retinoic acid receptor a. Reproduction 2010,139, 717-731. [CrossRef]
Gao, C.; Liao, M.Z.; Han, L.W.; Thummel, K.E.; Mao, Q. Hepatic Transport of 25-Hydroxyvitamin D. Drug Metab. Dispos. 2018, 46, 581-591. [CrossRef]
Chen, Y.; Liu, X.; Zhang, F.; Liao, S.; He, X.; Zhuo, D.; Huang, H.; Wu, Y. Vitamin D receptor suppresses proliferation and metastasis in renal cell carcinoma cell lines via regulating the expression of the epithelial Ca2+ channel TRPV5. PLoS ONE 2018, 13, e0195844. [CrossRef]
Long, W.; Fatehi, M.; Soni, S.; Panigrahi, R.; Philippaert, K.; Yu, Y.; Kelly, R.; Boonen, B.; Barr, A.; Golec, D.; et al. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J. Physiol. 2020, 598, 4321-4338. [CrossRef] [PubMed]
Long, W.; Johnson, J.; Kalyaanamoorthy, S.; Light, P. TRPV1 channels as a newly identified target for vitamin D. Channels 2021,15, 360-374. [CrossRef] [PubMed]
Callejo, M.; Mondejar-Parreño, G.; Morales-Cano, D.; Barreira, B.; Esquivel-Ruiz, S.; Olivencia, M.A.; Manaud, G.; Perros, F.; Duarte, J.; Moreno, L.; et al. Vitamin D deficiency downregulates TASK-1 channels and induces pulmonary vascular dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L627-L640. [CrossRef] [PubMed]
García-Becerra, R.; Díaz, L.; Camacho, J.; Barrera, D.; Ordaz-Rosado, D.; Morales, A.; Ortiz, C.S.; Avila, E.; Bargallo, E.; Arrecillas, M.; et al. Calcitriol inhibits Ether-a go-go potassium channel expression and cell proliferation in human breast cancer cells. Exp. Cell Res. 2010, 316, 433-442. [CrossRef]
Avila, E.; García-Becerra, R.; Rodríguez-Rasgado, J.A.; Díaz, L.; Ordaz-Rosado, D.; Zügel, U.; Steinmeyer, A.; Barrera, D.; Halhali, A.; Larrea, F.; et al. Calcitriol down-regulates human ether a go-go 1 potassium channel expression in cervical cancer cells. Anticancer Res. 2010, 30, 2667-2672.
Tripathy, B.; Majhi, R.K. TRPV1 channel as the membrane vitamin D receptor: Solving part of the puzzle. J. Physiol. 2020, 598, 5601-5603. [CrossRef] [PubMed]
Tamayo, M.; Manzanares, E.; Bas, M.; Martín-Nunes, L.; Val-Blasco, A.; Jesús Larriba, M.; Fernández-Velasco, M.; Delgado, C. Calcitriol (1,25-dihydroxyvitamin D. Heart Rhythm 2017,14, 432-439. [CrossRef] [PubMed]
Tamayo, M.; Martin-Nunes, L.; Val-Blasco, A.; Piedras, M.J.; Larriba, M.J.; Gómez-Hurtado, N.; Fernández-Velasco, M.; Delgado, C. Calcitriol, the Bioactive Metabolite of Vitamin D, Increases Ventricular K+ Currents in Isolated Mouse Cardiomyocytes. Front. Physiol. 2018, 9,1186. [CrossRef]
Sequeira, V.B.; Rybchyn, M.S.; Gordon-Thomson, C.; Tongkao-On, W.; Mizwicki, M.T.; Norman, A.W.; Reeve, V.E.; Halliday, G.M.; Mason, R.S. Opening of chloride channels by 1 a,25-dihydroxyvitamin D3 contributes to photoprotection against UVR-induced thymine dimers in keratinocytes. J. Investig. Dermatol. 2013,133, 776-782. [CrossRef]
Olszewska, A.M.; Sieradzan, A.K.; Bednarczyk, P.; Szewczyk, A.; Zmijewski, M.A. Mitochondrial potassium channels: A novel calcitriol target. Cell. Mol. Biol. Lett. 2022, 27, 3. [CrossRef] [PubMed]
Chen, J.; Doroudi, M.; Cheung, J.; Grozier, A.L.; Schwartz, Z.; Boyan, B.D. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1a,25(OH)(2)D(3). Cell. Signal. 2013, 25,2362-2373. [CrossRef] [PubMed]
Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018,19,1672. [CrossRef] [PubMed]
Silvagno, F.; Consiglio, M.; Foglizzo, V.; Destefanis, M.; Pescarmona, G. Mitochondrial translocation of vitamin D receptor is mediated by the permeability transition pore in human keratinocyte cell line. PLoS ONE 2013, 8, e54716. [CrossRef]
Silvagno, F.; De Vivo, E.; Attanasio, A.; Gallo, V.; Mazzucco, G.; Pescarmona, G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS ONE 2010, 5, e8670. [CrossRef]
Filipovic, N.; Bocina, I.; Restovic, I.; Grobe, M.; Kretzschmar, G.; Kevic, N.; Masek, T.; Vitlov Uljevic, M.; Juric, M.; Vukojevic, K.; et al. Ultrastructural characterization of vitamin D receptors and metabolizing enzymes in the lipid droplets of the fatty liver in rat. Acta Histochem. 2020,122,151502. [CrossRef]
Mizwicki, M.T.; Menegaz, D.; Yaghmaei, S.; Henry, H.L.; Norman, A.W. A molecular description of ligand binding to the two overlapping binding pockets of the nuclear vitamin D receptor (VDR): Structure-function implications. J. Steroid Biochem. Mol. Biol. 2010, 121, 98-105. [CrossRef]
Tapia, C.; Suares, A.; De Genaro, P.; González-Pardo, V. In vitro studies revealed a downregulation of Wnt/ß-catenin cascade by active vitamin D and TX 527 analog in a Kaposi's sarcoma cellular model. Toxicol. In Vitro 2020, 63,104748. [CrossRef] [PubMed]
Muralidhar, S.; Filia, A.; Nsengimana, J.; Pozniak, J.; O'Shea, S.J.; Diaz, J.M.; Harland, M.; Randerson-Moor, J.A.; Reichrath, J.; Laye, J.P.; et al. Vitamin D-VDR Signaling Inhibits Wnt/ß-Catenin-Mediated Melanoma Progression and Promotes Antitumor Immunity. Cancer Res. 2019, 79, 5986-5998. [CrossRef] [PubMed]
Tang, L.; Fang, W.; Lin, J.; Li, J.; Wu, W.; Xu, J. Vitamin D protects human melanocytes against oxidative damage by activation of Wnt/ß-catenin signaling. Lab. Investig. 2018, 98,1527-1537. [CrossRef] [PubMed]
Larriba, M.J.; Gonzalez-Sancho, J.M.; Bonilla, F.; Munoz, A. Interaction of vitamin D with membrane-based signaling pathways. Front. Physiol. 2014, 5, 60. [CrossRef]
Bikle, D.D.; Jiang, Y.; Nguyen, T.; Oda, Y.; Tu, C.L. Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front. Physiol. 2016, 7, 296. [CrossRef]
Bandera Merchan, B.; Morcillo, S.; Martin-Nuñez, G.; Tinahones, F.J.; Macías-González, M. The role of vitamin D and VDR in carcinogenesis: Through epidemiology and basic sciences. J. Steroid Biochem. Mol. Biol. 2017,167, 203-218. [CrossRef]
Hadden, M.K. Hedgehog and Vitamin D Signaling Pathways in Development and Disease. Vitam. Horm. 2016,100, 231-253. [CrossRef]
Lisse, T.S.; Saini, V.; Zhao, H.; Luderer, H.F.; Gori, F.; Demay, M.B. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol. Endocrinol. 2014, 28,1698-1706. [CrossRef]
Teichert, A.E.; Elalieh, H.; Elias, P.M.; Welsh, J.; Bikle, D.D. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J. Investig. Dermatol. 2011,131, 2289-2297. [CrossRef]
Wang, H.; Wang, X.; Xu, L.; Zhang, J.; Cao, H. A molecular sub-cluster of colon cancer cells with low VDR expression is sensitive to chemotherapy, BRAF inhibitors and PI3K-mTOR inhibitors treatment. Aging 2019,11, 8587-8603. [CrossRef] [PubMed]
Olsson, K.; Saini, A.; Stromberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1a,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98-111. [CrossRef] [PubMed]
Singh, P.K.; van den Berg, P.R.; Long, M.D.; Vreugdenhil, A.; Grieshober, L.; Ochs-Balcom, H.M.; Wang, J.; Delcambre, S.; Heikkinen, S.; Carlberg, C.; et al. Integration of VDR genome wide binding and GWAS genetic variation data reveals cooccurrence of VDR and NF-kB binding that is linked to immune phenotypes. BMC Genom. 2017,18,132. [CrossRef]
Doroudi, M.; Boyan, B.D.; Schwartz, Z. Rapid 1a,25(OH)2D3 membrane-mediated activation of Ca2+/calmodulin-dependent protein kinase II in growth plate chondrocytes requires Pdia3, PLAA and caveolae. Connect. Tissue Res. 2014, 55 (Suppl. S1), 125-128. [CrossRef]
Doroudi, M.; Schwartz, Z.; Boyan, B.D. Phospholipase A2 activating protein is required for 1a,25-dihydroxyvitamin D3 dependent rapid activation of protein kinase C via Pdia3. J. Steroid Biochem. Mol. Biol. 2012,132, 48-56. [CrossRef]
Chignell, C.F.; Kukielczak, B.M.; Sik, R.H.; Bilski, P.J.; He, Y.Y. Ultraviolet A sensitivity in Smith-Lemli-Opitz syndrome: Possible involvement of cholesta-5,7,9(11)-trien-3 beta-ol. Free Radic. Biol. Med. 2006, 41, 339-346. [CrossRef] [PubMed]
De Fabiani, E.; Caruso, D.; Cavaleri, M.; Galli Kienle, M.; Galli, G. Cholesta-5,7,9(11)-trien-3 beta-ol found in plasma of patients with Smith-Lemli-Opitz syndrome indicates formation of sterol hydroperoxide. J. Lipid Res. 1996, 37, 2280-2287. [CrossRef]
Jin, X.; Yang, X.; Yang, L.; Liu, Z.-L.; Zhang, F. Autoxidation of isotachysterol. Tetrahedron 2004, 60, 2881-2888. [CrossRef]
Zmijewski, M.A.; Li, W.; Chen, J.; Kim, T.K.; Zjawiony, J.K.; Sweatman, T.W.; Miller, D.D.; Slominski, A.T. Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids 2011, 76,193-203. [CrossRef]
Guo, L.W.; Wilson, W.K.; Pang, J.; Shackleton, C.H. Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17alpha,20- triols, potential steroid metabolites in Smith-Lemli-Opitz syndrome. Steroids 2003, 68, 31-42. [CrossRef]
Valencia, A.; Kochevar, I.E. Ultraviolet A induces apoptosis via reactive oxygen species in a model for Smith-Lemli-Opitz syndrome. Free Radic. Biol. Med. 2006, 40, 641-650. [CrossRef] [PubMed]
Jablonski, N.G.; Chaplin, G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int. J. Paleopathol. 2018, 23, 54-59. [CrossRef] [PubMed]
de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996,15-23. [CrossRef] [PubMed]
Ravanat, J.L.; Douki, T.; Cadet, J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B 2001, 63, 88-102. [CrossRef] [PubMed]
De Silva, W.G.M.; Han, J.Z.R.; Yang, C.; Tongkao-On, W.; McCarthy, B.Y.; Ince, F.A.; Holland, A.J.A.; Tuckey, R.C.; Slominski, A.T.; Abboud, M.; et al. Evidence for Involvement of Nonclassical Pathways in the Protection from UV-Induced DNA Damage by Vitamin D-Related Compounds. JBMR Plus 2021, 5, e10555. [CrossRef] [PubMed]
Lin, Y.; Cao, Z.; Lyu, T.; Kong, T.; Zhang, Q.; Wu, K.; Wang, Y.; Zheng, J. Single-cell RNA-seq of UVB-radiated skin reveals landscape of photoaging-related inflammation and protection by vitamin D. Gene 2022, 831,146563. [CrossRef]
Reichrath, J.; Rass, K. Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: An update. Adv. Exp. Med. Biol. 2014, 810, 208-233.
Scott, J.F.; Das, L.M.; Ahsanuddin, S.; Qiu, Y.; Binko, A.M.; Traylor, Z.P.; Debanne, S.M.; Cooper, K.D.; Boxer, R.; Lu, K.Q. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J. Investig. Dermatol. 2017,137, 2078-2086. [CrossRef]
Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D'Orazio, J.A.; Holick, M.F; Tang, E.K.Y.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D. Redox Biol. 2019, 24,101206. [CrossRef]
Donati, S.; Palmini, G.; Aurilia, C.; Falsetti, I.; Miglietta, F.; Iantomasi, T.; Brandi, M.L. Rapid Nontranscriptional Effects of Calcifediol and Calcitriol. Nutrients 2022,14,1291. [CrossRef]
Janjusevic, M.; Gagno, G.; Fluca, A.L.; Padoan, L.; Beltrami, A.P.; Sinagra, G.; Moretti, R.; Aleksova, A. The peculiar role of vitamin D in the pathophysiology of cardiovascular and neurodegenerative diseases. Life Sci. 2022,289,120193. [CrossRef] [PubMed]
Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; Deluca, H.F. CYP2R1 is a major, but not exclusive, contributor to 25- hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA 2013,110,15650-15655. [CrossRef] [PubMed]
Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015,151, 25-37. [CrossRef] [PubMed]
Tuckey, R.C.; Li, W.; Ma, D.; Cheng, C.Y.S.; Wang, K.M.; Kim, T.K.; Jeayeng, S.; Slominski, A.T. CYP27A1 acts on the pre-vitamin D3 photoproduct, lumisterol, producing biologically active hydroxy-metabolites. J. Steroid Biochem. Mol. Biol. 2018,181,1-10. [CrossRef] [PubMed]
Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5,14875. [CrossRef]
Slominski, A.T.; Janjetovic, Z.; Fuller, B.E.; Zmijewski, M.A.; Tuckey, R.C.; Nguyen, M.N.; Sweatman, T.; Li, W.; Zjawiony, J.; Miller, D.; et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS ONE 2010, 5, e9907. [CrossRef]
Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78,165-180. [CrossRef]
Quigley, M.; Rieger, S.; Capobianco, E.; Wang, Z.; Zhao, H.; Hewison, M.; Lisse, T.S. Vitamin D Modulation of Mitochondrial Oxidative Metabolism and mTOR Enforces Stress Adaptations and Anticancer Responses. JBMR Plus 2022, 6, e10572. [CrossRef]
Consiglio, M.; Destefanis, M.; Morena, D.; Foglizzo, V.; Forneris, M.; Pescarmona, G.; Silvagno, F. The vitamin D receptor inhibits the respiratory chain, contributing to the metabolic switch that is essential for cancer cell proliferation. PLoS ONE 2014, 9, e115816. [CrossRef]
Demonacos, C.V.; Karayanni, N.; Hatzoglou, E.; Tsiriyiotis, C.; Spandidos, D.A.; Sekeris, C.E. Mitochondrial genes as sites of primary action of steroid hormones. Steroids 1996, 61, 226-232. [CrossRef]
Mersa, A.; Atashbar, S.; Ahvar, N.; Salimi, A. 1,25-dihydroxyvitamin D3 prevents deleterious effects of erythromycin on mitochondrial function in rat heart isolated mitochondria. Clin. Exp. Pharmacol. Physiol. 2020, 47,1554-1563. [CrossRef] [PubMed]
Mirzakhani, H.; Litonjua, A.A.; McElrath, T.F.; O'Connor, G.; Lee-Parritz, A.; Iverson, R.; Macones, G.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.; et al. Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Investig. 2016,126, 4702-4715. [CrossRef] [PubMed]
Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [CrossRef] [PubMed]
Pilz, S.; Theiler-Schwetz, V.; Pludowski, P.; Zelzer, S.; Meinitzer, A.; Karras, S.N.; Misiorowski, W.; Zittermann, A.; März, W.; Trummer, C. Hypercalcemia in Pregnancy Due to CYP24A1 Mutations: Case Report and Review of the Literature. Nutrients 2022, 14, 2518. [CrossRef] [PubMed]
Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876-884. [CrossRef]
Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018,13,1177271918755391. [CrossRef] [PubMed]
Berridge, M.J. Vitamin D: A custodian of cell signalling stability in health and disease. Biochem. Soc. Trans. 2015, 43, 349-358. [CrossRef]
Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D . J. Steroid Biochem. Mol. Biol. 2018,175, 60-81. [CrossRef]
Sultan, M.; Twito, O.; Tohami, T.; Ramati, E.; Neumark, E.; Rashid, G. Vitamin D diminishes the high platelet aggregation of type 2 diabetes mellitus patients. Platelets 2019, 30,120-125. [CrossRef]
Gonzalez-Sanchez, E.; El Mourabit, H.; Jager, M.; Clavel, M.; Moog, S.; Vaquero, J.; Ledent, T.; Cadoret, A.; Gautheron, J.; Fouassier, L.; et al. Cholangiopathy aggravation is caused by VDR ablation and alleviated by VDR-independent vitamin D signaling in ABCB4 knockout mice. Biochim. Biophys. Acta Mol. Basis Dis. 2021,1867,166067. [CrossRef]
Liu, Y.; Wang, J.X.; Nie, Z.Y.; Wen, Y.; Jia, X.J.; Zhang, L.N.; Duan, H.J.; Shi, Y.H. Upregulation of ERp57 promotes clear cell renal cell carcinoma progression by initiating a STAT3/ILF3 feedback loop. J. Exp. Clin. Cancer Res. 2019, 38, 439. [CrossRef] [PubMed]
Janjetovic, Z.; Zmijewski, M.A.; Tuckey, R.C.; DeLeon, D.A.; Nguyen, M.N.; Pfeffer, L.M.; Slominski, A.T. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS ONE 2009, 4, e5988. [CrossRef]
Slominski, A.T.; Kim, T.K.; Slominski, R.M.; Song, Y.; Janjetovic, Z.; Podgorska, E.; Reddy, S.B.; Raman, C.; Tang, E.K.Y.; Fabisiak, A.; et al. Metabolic activation of tachysterol. FASEB J. 2022, 36, e22451. [CrossRef]
Carlberg, C. The physiology of vitamin D-far more than calcium and bone . Front. Physiol. 2014, 5, 335. [CrossRef]
Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D's Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 17685. [CrossRef]
Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022,14, 3811. [CrossRef]
{include}
