Sickle cell and low vitamin D – 3 presentations
All presented at 2012 American Society of Hematology December 8-11, 2012 Atlanta, GA
Vitamin D and Zinc Deficiency Associated with Increased Pain Episodes in Children with Sickle Cell Disease: poster, Abstract 3237
Abi Vijenthira, BHSc,1, Christine G Chretien, BSCN,2, Sydney Harris-Janz, RD*,2 and Robert J Klaassen, MD2
1 Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada,
2 Division of Hematology/Oncology, Children's Hospital of Eastern Ontario, Ottawa, ON, Canada
Background: Sickle cell disease (SCD) is characteristically described as a disease of hemolytic anemia and vaso-occlusive crises (VOC). However, patients suffer from a multitude of other problems including impaired growth and development, chronic pain, increased susceptibility to infection, poor bone health, and impaired cell-mediated immunity. Nutritional deficiency has been implicated as a contributor to these issues. The objective of this study was to provide a comprehensive overview of the nutrition status of pediatric SCD patients, and test for previously studied associations between nutrients and markers of disease severity and growth.
Methods: Retrospective cross-sectional pilot study at a Canadian pediatric tertiary care centre from March to June 2012. Patients with all SCD genotypes who were between 2–18 years were eligible. As part of a routine follow-up clinic visit, patients were tested for serum levels of vitamin D (calcidiol and calcitriol), zinc, B6, B12, folate, and homocysteine. Note that all patients were prescribed folic acid supplementation. Information regarding laboratory values, growth, and markers of disease severity was obtained via medical record abstraction. Disease severity was categorized as mild (healthy patients with no hospital visits in past 12 months), moderate (emergency department (ED) visits related to SCD in past 12 months, home narcotic usage, and/or hydroxyurea usage) or severe (hospitalizations related to SCD in past 12 months, transfusion requirement, and/or end-organ damage (e.g. retinopathy)). Total nutrient deficiencies were categorized into none, single, or multiple.
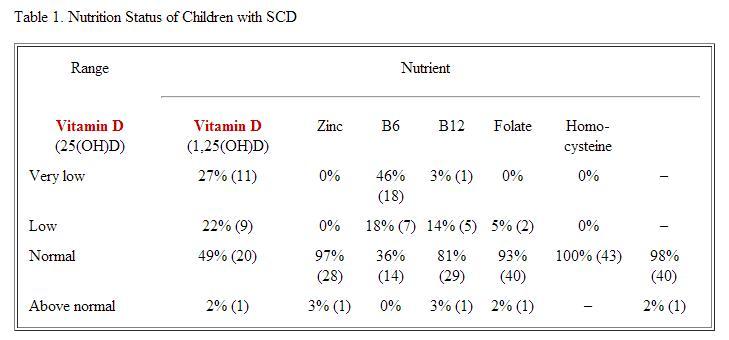
Results: Preliminary analysis of 43 patients was conducted (11.2 + 4.1 yrs, 40% female). Half the sample (50%) had multiple nutrient deficiencies, and 31% had a single deficiency. Deficiencies were seen most frequently in calcidiol (25(OH)D) (49% with insufficiency) and zinc (64% with deficiency), although there was no association between them (r=0.1, p=0.4). See Table 1 for an overview of nutrition status of all patients. Of all the markers of growth and disease severity tested, VOC was significantly associated with both vitamin D (calcidiol) deficiency and zinc deficiency. More specifically, calcidiol deficiency was moderately and significantly associated with total number of pain episodes (hospital, ED, and home) over the past 12 months (r=–0.4, p=0.02). Zinc deficiency was moderately and significantly associated with number of pain episodes managed at home over the past 12 months (r=–0.34, p=0.04).
Other variables showed no significant findings. There were no gender differences in number of deficiencies (p=0.2). In terms of growth, patients had normal mean height-for-age (45.6 + 29.1 percentile) and weight-for-age (47.8 + 25.7 percentile). There was no association between disease severity and height-for-age (r=–0.2, p=0.2), weight-for-age (r=–0.1, p=0.6), or number of deficiencies (r=0.2, p=0.4). Calcidiol deficiency was not associated with height-for-age (r=0.6, p=0.7). Zinc deficiency was not associated with height-for-age (r=0.05, p=0.8), or with number of hospitalizations for VOC (r=–0.26, p=0.1) or infection (r=–0.1, p=0.6) in the past 12 months.
Conclusions: This is the first study to provide a more complete overview of nutrition status in children with SCD. The majority of patients had multiple nutrient deficiencies, primarily in vitamin D (calcidiol) and zinc. Calcidiol deficiency was associated with increased VOC. Finally, this study contributes to growing evidence for the link between zinc's anti-sickling properties and reduced VOC, although we only found a significant association for home pain crises; this may be due to the low overall rate of hospitalizations in our sample. Prospective studies with larger samples are needed to further elucidate the relationship between nutrient deficiencies and SCD, and to determine whether nutrient supplementation can improve the disease course.
Relationship Between vitamin D Deficiency and Bone Fragility in Sickle Cell Disease:
A Cohort Study of 56 adults Poster Abstract 2103
Jean-Benoît Arlet, MD,1, Marie Courbebaisse, MD, PhD,2, Gilles Chatellier, MD,3, Dominique Eladari, MD, PhD,2, Jean-Claude Souberbielle, MD,4, Gerard Friedlander, MD, PhD,2, Mariane De Montalembert, MD5, Dominique Prié, MD, PhD,6, Jacques Pouchot, MD,1 and Jean-Antoine Ribeil, MD, PhD*,7
1 Service de médecine interne, Hôpital européen Georges Pompidou, Paris, France,
2 Service d'explorations fonctionnelles et physiologiques, Hôpital Necker, Paris, France,
3 CIC-EC4 INSERM, Hôpital européen Georges Pompidou, Paris, France,
4 Service d'explorations fonctionnelles et physiologiques, Hôpital Necker, Paris,
5 Service de Pediatrie, Hospital Necker, Paris, France,
6 Service de pédiatrie générale, Hôpital Necker, Paris, France,
7 Biotherapie, Hôpital Necker, Paris, France
Background: Recent studies suggest that patients with sickle cell disease (SCD) have profound vitamin D (VD) deficiency. Limited data exist on the effect of VD deficiency on bone fragility in these patients.
Objectives: To assess the prevalence of VD deficiency in adults with SCD and its consequences on bone metabolism and fragility.
Methods: This prospective study included 56 SCD adult patients (mean age 29.8 ± 9.5 years), in a clinically steady state. Clinical and laboratory data were recorded. Bone mineral density (BMD) was measured using dual X-ray absorptiometry. Fracture history, BMD, avascular osteonecrosis, H-shaped vertebra and markers of mineral metabolism were compared between two groups of patients presenting very low (6 ng/ml, n=26) (group 1) and low (>6 ng/ml, n=26) (group 2) 25(OH)D concentration, respectively.
Results: Median 25(OH)D concentration was 6 ng/mL. VD deficiency (25(OH)D <10 ng/mL) was found in 42 out of 56 patients (75%) and secondary hyperparathyroidism in 40 (71.4%). History of fracture was documented in 17 patients (30.3%), osteopenia and/or ospeoporosis in 39.6% of patients. Overall, patients of group 1 were more likely to have sustained a fracture (42.8%) compared to patients of group 2 (17.8%) (p=0.04). These patients had also lower body mass index and significantly higher parathyroid hormone, C-terminal telopeptides of type I-collagen and bone-specific alkaline phosphatase serum levels. There was no difference between group for BMD, avascular osteonecrosis history, H-shaped vertebra, and disease severity markers.
Conclusion: This study suggests that VD deficiency is a key feature in SCD-bone disease. It is highly prevalent and associated with hyperparathyroidism, bone resorption markers, and history of fracture. The optimal supplementation regimen remains to be determined.
Vitamin Deficiency, CD40L and VEGF in a Young Population with Sickle Cell Disease, Abstract 4768
E. Leila Jerome Clay, MD1, Julia Brittain, PhD*,2 and Rupa Redding-Lallinger, MD3
1 Department of Pediatrics, Division of Hematology/Oncology, University of North Carolina School of Medicine, Chapel Hill, NC, USA,
2 Georgia Health Sciences University, Augusta, GA, USA,
3 Departments of Pediatrics & Internal Medicine, Division of Hematology /Oncology, University of North Carolina School of Medicine, Chapel Hill, NC, USA
Introduction: Vitamin D deficiency is known to be common in patients with sickle cell disease (SCD). Vitamin D is important in multiple aspects of health, including the cardiovascular, immune and skeletal systems and its effects are mediated through the vitamin D receptor. The systems affected by vitamin D are also perturbed by SCD. Vitamin D deficiency is common in SCD, but its contribution to disease manifestations is being investigated. Vitamin D modulates the immune response and may have an effect on the levels of increased inflammation seen in individuals with SCD. In children and young adults with SCD at UNC Hospitals, we sought to determine the prevalence of vitamin D deficiency and its association with inflammatory markers and the influence of VDR haplotype. We report here on vitamin D status and several markers of inflammation.
Methods: We recruited pediatric and young adult SCD patients in their steady state attending routine periodic evaluations at the Pediatric and Adult Sickle Cell Clinics at the University of North Carolina Hospitals between February and June 2012. After consent, patients had their blood collected for inflammatory markers, 25-OH vitamin D and DNA. Patients with active pain crisis or recent illness were excluded. A chart review was done for the last 5 years to obtain SCD genotype, baseline white blood cell count, hemoglobin, platelets, calcium, phosphate and alkaline phosphatase. We measured inflammatory markers IL2, IL6, CD40L, TNFa, plasma VEGF and CD40L levels using ELISA (R&D; Systems). At present only the VEGF and CD40L levels, along with baseline clinical laboratory data are available with the other inflammatory marker data expected shortly. Spearman's regression was used to examine potential correlations between continuous variables. A p value of < 0.05 was considered significant. P-values are considered nominal and are uncorrected for multiple analyses.
Results: Vitamin D levels were measured in 78 patients, ages ranging from 2 to 26 years, with 55% males. The SCD genotypes were SS and Sb°Thal at 80%, SC and Sb+Thal at 20%. Thirty percent of patients were on hydroxyurea and ten percent of patients were on chronic exchange transfusions.
Severe vitamin D deficiency (<10 ng/mL) was present in 18%, mild to moderate deficiency (10–24 ng/mL) in 54% and only 28% were sufficient (>25 ng/mL). VEGF mean was 110.1 pg/mL (SD 125.8). CD40L mean was 642.2 pg/mL (SD 378.2). For the group as a whole, there were no correlations between the inflammatory markers and 25-OH vitamin D levels. However, when the group who was vitamin D deficient (< 25 ng/mL) was examined (n=39), vitamin D levels were inversely correlated with platelet count (rho= –0.3596, p =0.0246). Platelet count was positively correlated with CD40L level (rho= 0.3176, p= 0.0488). VEGF and CD40L levels were positively correlated (rho= 0.4520, p= 0.0039). Vitamin D levels are negatively correlated with age (rho= –0.3794, p = 0.0172). Restricting the analyses by age and gender did not change the results, nor did removing the individuals on hydroxyurea or chronic transfusions.
Discussion: As has been noted previously, vitamin D deficiency is very common in people with sickle cell disease, including this young population, with mean age of 14 years. Inflammation is common as well, as reflected by the markedly elevated CD40L levels as well as the high-normal distribution of white cell count and platelet count. VEGF levels in adults with SCD appear to be elevated although are quite variable; VEGF appears to be a marker of inflammation in this disease. Little is known about VEGF levels in children with SCD. No associations between vitamin D levels and CD40L or VEGF levels were seen, however an inverse correlation between vitamin D level and platelet count was found. As platelets are a marker of inflammation, this suggests that further investigation of the relationship between vitamin D deficiency and inflammation could be fruitful. We anticipate having data concerning vitamin D and IL2, IL6 and TNFa in this group in the near future, as well as the ability to stratify the individuals by VDR haplotype.
Disclosures: Redding-Lallinger: Eli Lilly and Company: Research Funding.
See also VitaminDWiki
Sickle Cell Anemia: 64 percent had less than 10ng of vitamin D – April 2012
Search VitaminDWiki for "sickle cell" 112 items as of March 2015
14000 IU vitamin D (50000 twice a week) often stops Sickle Cell pain
Hardly any Sickle Cell Anemia children had 30 ng of vitamin D – April 2012
Overview Dark Skin and Vitamin D - typically very low on vitamin D if not supplemented
Sickle cell Vitamin D deficiency corrected with 160 K IU loading dose – July 2014
Zinc and Vitamin D category listing has items along with related searches
See also PubMed
- Relationship between vitamin D deficiency and bone fragility in sickle cell disease: a cohort study of 56 adults. Jan 2013
- Median = 6 ng of Vitamin D. 43% of those < 6ng had a fracture, vs 18% if > 6 ng
- An update on the recent literature on sickle cell bone disease Oct 2013
- A recent study demonstrated reduced days with pain and improved physical activity quality of life following high-dose vitamin D therapy
