Fertility is a function of vitamin D around each egg (follicular fluid) – many studies
Low vitamin D levels in follicular fluid, but not in serum, are associated with adverse outcomes in assisted reproduction - Feb 2022
Archives of Gynecology and Obstetrics V 305, pages 505–517 https://doi.org/10.1007/s00404-021-06174-9
Kahindo P. Muyayalo, Su Song, Hui Zhai, Hong Liu, Dong-Hui Huang, Hui Zhou, Yang-Jiao Chen & Ai-Hua Liao
Purpose
To assess the relationship between serum/follicular fluid (FF) vitamin D (VD) status and assisted reproductive technology (ART) treatment outcomes among infertile patients.
Methods
A prospective cohort study, including 132 infertile patients scheduled for their first ART treatment cycle, was carried out in a Reproductive Medical Center. Serum and FF samples were collected to assess 25-hydroxy VD [25(OH)D] levels. Low VD level was defined as 25(OH)D concentration of less than 30 ng/mL.
Results
Most infertile patients had low VD levels in serum (88%) and FF (90%). We observed a moderately positive correlation between VD levels in serum and FF (r = 0.34, p < 0.0001). Compared to the group of patients with low VD levels in the FF, those with sufficient VD levels had a significantly higher number of retrieved oocytes (p = 0.03), normal fertilization (p = 0.01), and high-quality embryos (p = 0.001). Moreover, patients with sufficient VD levels in the FF also had significantly higher implantation rates than those with low VD levels (76.92% vs. 46.58%, respectively, p = 0.01) and clinical pregnancy rates (92.31% vs. 61.54%, respectively, p = 0.04).
Conclusion
These data collectively revealed that low VD levels in serum and FF were common among infertile patients. VD levels in FF, but not in serum, were associated with embryo quality, normal fertilization, implantation rates, and clinical pregnancy rates. Further studies are mandatory to determine the molecular mechanism and VD’s potential therapeutic benefits in infertile patients.
References (4 on vit D in follicular fluid)
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G (2016) Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 96(1):365–408. https://doi.org/10.1152/physrev.00014.2015
Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L (2012) Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol 280(1):36–43. https://doi.org/10.1016/j.cellimm.2012.10.009
Van Etten E, Decallonne B, Verlinden L, Verstuyf A, Bouillon R, Mathieu C (2003) Analogs of 1alpha,25-dihydroxyvitamin D3 as pluripotent immunomodulators. J Cell Biochem 88(2):223–226. https://doi.org/10.1002/jcb.10329
Irani M, Merhi Z (2014) Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril 102(2):460–8 e3. https://doi.org/10.1016/j.fertnstert.2014.04.046
Du H, Daftary GS, Lalwani SI, Taylor HS (2005) Direct regulation of HOXA10 by 1,25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol 19(9):2222–2233. https://doi.org/10.1210/me.2004-0336
Wehr E, Pieber TR, Obermayer-Pietsch B (2011) Effect of vitamin D3 treatment on glucose metabolism and menstrual frequency in polycystic ovary syndrome women: a pilot study. J Endocrinol Invest 34(10):757–763. https://doi.org/10.3275/7748
Asadi M, Matin N, Frootan M, Mohamadpour J, Qorbani M, Tanha FD (2014) Vitamin D improves endometrial thickness in PCOS women who need intrauterine insemination: a randomized double-blind placebo-controlled trial. Arch Gynecol Obstet 289(4):865–870. https://doi.org/10.1007/s00404-013-3055-x
Lasco A, Catalano A, Benvenga S (2012) Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med 172(4):366–367. https://doi.org/10.1001/archinternmed.2011.715
Wise LA, Ruiz-Narvaez EA, Haddad SA, Rosenberg L, Palmer JR (2014) Polymorphisms in vitamin D-related genes and risk of uterine leiomyomata. Fertil Steril 102(2):503–10 e1. https://doi.org/10.1016/j.fertnstert.2014.04.037PubMed Central
Nair R, Maseeh A (2012) Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother 3(2):118–126. https://doi.org/10.4103/0976-500X.95506PubMed Central
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281. https://doi.org/10.1056/NEJMra070553
Burgaz A, Orsini N, Larsson SC, Wolk A (2011) Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens 29(4):636–645. https://doi.org/10.1097/HJH.0b013e32834320f9
Satirapoj B, Limwannata P, Chaiprasert A, Supasyndh O, Choovichian P (2013) Vitamin D insufficiency and deficiency with stages of chronic kidney disease in an Asian population. BMC Nephrol 14:206. https://doi.org/10.1186/1471-2369-14-206PubMed Central
Ota K, Dambaeva S, Han AR, Beaman K, Gilman-Sachs A, Kwak-Kim J (2014) Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum Reprod 29(2):208–219. https://doi.org/10.1093/humrep/det424
Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA et al (2012) IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab 97(4):1146–1152. https://doi.org/10.1210/jc.2011-2218PubMed Central
Kebapcilar AG, Kulaksizoglu M, Kebapcilar L, Gonen MS, Unlu A, Topcu A et al (2013) Is there a link between premature ovarian failure and serum concentrations of vitamin D, zinc, and copper? Menopause 20(1):94–99. https://doi.org/10.1097/gme.0b013e31826015ca
Grundmann M, von Versen-Hoynck F (2011) Vitamin D—roles in women’s reproductive health? Reprod Biol Endocrinol 9:146. https://doi.org/10.1186/1477-7827-9-146PubMed Central
Ranjana H (2017) Role of vitamin D in infertility. J Public Health Policy Plann. 1:8–10
Human Fertilisation and Embryology Authority (HFEA). Fertility treatment in 2013: trends and figures. 2016. https://www.hfea.gov.uk/media/2081/hfea-fertility-trends-2013.pdf. Accessed 5 Jan 2020
Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2015 Assisted Reproductive Technology National Summary Report. Atlanta (GA): US Dept of Health and Human Services; 2017. https://www.cdc.gov/art/pdf/2015-report/art-2015-national-summary-report.pdf. Accessed 28 Mar 2020
Chu J, Gallos I, Tobias A, Tan B, Eapen A, Coomarasamy A (2018) Vitamin D and assisted reproductive treatment outcome: a systematic review and meta-analysis. Hum Reprod 33(1):65–80. https://doi.org/10.1093/humrep/dex326
Ciepiela P, Duleba AJ, Kowaleczko E, Chelstowski K, Kurzawa R (2018) Vitamin D as a follicular marker of human oocyte quality and a serum marker of in vitro fertilization outcome. J Assist Reprod Genet 35(7):1265–1276. https://doi.org/10.1007/s10815-018-1179-4
Hornstein MD (2019) Vitamin D and infertility: the evidence . Fertility Reprod 1(1):31–33
Lv SS, Wang JY, Wang XQ, Wang Y, Xu Y (2016) Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet 293(6):1339–1345. https://doi.org/10.1007/s00404-016-4058-1
Albuquerque LE, Saconato H, Maciel MC (2005) Depot versus daily administration of gonadotrophin releasing hormone agonist protocols for pituitary desensitization in assisted reproduction cycles. Cochrane Database Syst Rev 1:CD002808. https://doi.org/10.1002/14651858.CD002808.pub2
Xiao Y, Wang Y, Wang M, Liu K (2018) Follicular flushing increases the number of oocytes retrieved in poor ovarian responders undergoing in vitro fertilization: a retrospective cohort study. BMC Womens Health 18(1):186. https://doi.org/10.1186/s12905-018-0681-2PubMed Central
Brinsden PR (1999) A textbook of in vitro fertilization and assisted reproduction: the bourn hall guide to clinical and laboratory practice, 2nd edn. CRC Press, London
Liu X, Zhang W, Xu Y, Chu Y, Wang X, Li Q et al (2019) Effect of vitamin D status on normal fertilization rate following in vitro fertilization. Reprod Biol Endocrinol 17(1):59. https://doi.org/10.1186/s12958-019-0500-0PubMed Central
Abuzeid MI, Bolonduro O, La Chance J, Abozaid T, Urich M, Ullah K et al (2014) Cumulative live birth rate and assisted reproduction: impact of female age and transfer day. Facts Views Vis Obgyn 6(3):145–149
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930. https://doi.org/10.1210/jc.2011-0385
Muyayalo KP, Huang XB, Qian Z, Li ZH, Mor G, Liao AH (2019) Low circulating levels of vitamin D may contribute to the occurrence of preeclampsia through deregulation of Treg /Th17 cell ratio. Am J Reprod Immunol 82(4):e13168. https://doi.org/10.1111/aji.13168
Chu J, Gallos I, Tobias A, Robinson L, Kirkman-Brown J, Dhillon-Smith R et al (2019) Vitamin D and assisted reproductive treatment outcome: a prospective cohort study. Reprod Health 16(1):106. https://doi.org/10.1186/s12978-019-0769-7PubMed Central
Firouzabadi RD, Rahmani E, Rahsepar M, Firouzabadi MM (2014) Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch Gynecol Obstet 289(1):201–206. https://doi.org/10.1007/s00404-013-2959-9
Boz I, Teskereci G, Ozekinci M (2020) High prevalence of vitamin D deficiency in Turkish women undergoing in vitro fertilization: a descriptive study. Health Care Women Int 41(2):147–158. https://doi.org/10.1080/07399332.2019.1569015
Xie Q-W, Zhang M (2013) White or tan? A cross-cultural analysis of skin beauty advertisements between China and the United States. Asian J Commun 23(5):538–554. https://doi.org/10.1080/01292986.2012.756046
Zhang W, Stoecklin E, Eggersdorfer M (2013) A glimpse of vitamin D status in Mainland China. Nutrition 29(7–8):953–957. https://doi.org/10.1016/j.nut.2013.01.010
Aleyasin A, Hosseini MA, Mahdavi A, Safdarian L, Fallahi P, Mohajeri MR et al (2011) Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol 159(1):132–137. https://doi.org/10.1016/j.ejogrb.2011.07.006
Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C et al (2010) Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril 94(4):1314–1319. https://doi.org/10.1016/j.fertnstert.2009.05.019
Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S et al (2010) Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol 8:91. https://doi.org/10.1186/1477-7827-8-91PubMed Central
Mnallah S, Kacem Berjeb K, Braham M, Khrouf M, Chtourou S, Merdassi G et al (2017) Impact of vitamin D deficiency on ICSI outcomes. JFIV Reprod Med Genet. 5:201. https://doi.org/10.4172/2375-4508.1000201
Fabris A, Pacheco A, Cruz M, Puente JM, Fatemi H, Garcia-Velasco JA (2014) Impact of circulating levels of total and bioavailable serum vitamin D on pregnancy rate in egg donation recipients. Fertil Steril 102(6):1608–1612. https://doi.org/10.1016/j.fertnstert.2014.08.030
Franasiak JM, Molinaro TA, Dubell EK, Scott KL, Ruiz AR, Forman EJ et al (2015) Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol 212(3):315 e1-315 e6. https://doi.org/10.1016/j.ajog.2014.09.029
Neville G, Martyn F, Kilbane M, O’Riordan M, Wingfield M, McKenna M et al (2016) Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int J Gynaecol Obstet 135(2):172–176. https://doi.org/10.1016/j.ijgo.2016.04.018
Abadia L, Gaskins AJ, Chiu YH, Williams PL, Keller M, Wright DL et al (2016) Serum 25-hydroxyvitamin D concentrations and treatment outcomes of women undergoing assisted reproduction. Am J Clin Nutr 104(3):729–735. https://doi.org/10.3945/ajcn.115.126359PubMed Central
Merhi Z, Doswell A, Krebs K, Cipolla M (2014) Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab 99(6):E1137–E1145. https://doi.org/10.1210/jc.2013-4161PubMed Central
Cunningham TK, Allgar V, Dargham SR, Kilpatrick E, Sathyapalan T, Maguiness S et al (2019) Association of vitamin D metabolites with embryo development and fertilization in women with and without PCOS undergoing subfertility treatment. Front Endocrinol (Lausanne) 10:13. https://doi.org/10.3389/fendo.2019.00013
Farzadi L, Khayatzadeh Bidgoli H, Ghojazadeh M, Bahrami Z, Fattahi A, Latifi Z et al (2015) Correlation between follicular fluid 25-OH vitamin D and assisted reproductive outcomes. Iran J Reprod Med 13(6):361–366
Paffoni A, Ferrari S, Vigano P, Pagliardini L, Papaleo E, Candiani M et al (2014) Vitamin D deficiency and infertility: insights from in vitro fertilization cycles. J Clin Endocrinol Metab 99(11):E2372–E2376. https://doi.org/10.1210/jc.2014-1802
Garbedian K, Boggild M, Moody J, Liu KE (2013) Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open 1(2):E77-82. https://doi.org/10.9778/cmajo.20120032
Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA (2014) Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril 101(2):447–452. https://doi.org/10.1016/j.fertnstert.2013.10.008
Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K et al (2013) Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod 19(8):519–527. https://doi.org/10.1093/molehr/gat024
Arefi S, Khalili G, Iranmanesh H, Farifteh F, Hosseini A, Fatemi HM et al (2018) Is the ovarian reserve influenced by vitamin D deficiency and the dress code in an infertile Iranian population? J Ovarian Res 11(1):62. https://doi.org/10.1186/s13048-018-0435-7
Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L (2001) Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 167(4):1945–1953. https://doi.org/10.4049/jimmunol.167.4.1945
Velthut A, Zilmer M, Zilmer K, Kaart T, Karro H, Salumets A (2013) Elevated blood plasma antioxidant status is favourable for achieving IVF/ICSI pregnancy. Reprod Biomed Online 26(4):345–352. https://doi.org/10.1016/j.rbmo.2012.12.012
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37(4):277–285. https://doi.org/10.1016/j.clinbiochem.2003.11.015
Agarwal A, Gupta S, Sharma RK (2005) Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 3:28. https://doi.org/10.1186/1477-7827-3-28
Dalto DB, Matte JJ (2017) Pyridoxine (vitamin B(6)) and the glutathione peroxidase system; a link between one-carbon metabolism and antioxidation. Nutrients. https://doi.org/10.3390/nu9030189
Nunez-Calonge R, Cortes S, Gutierrez Gonzalez LM, Kireev R, Vara E, Ortega L et al (2016) Oxidative stress in follicular fluid of young women with low response compared with fertile oocyte donors. Reprod Biomed Online 32(4):446–456. https://doi.org/10.1016/j.rbmo.2015.12.010
Olszak-Wąsik K, Bednarska-Czerwińska A, Olejek A, Tukiendorf A (2019) From, “every day” hormonal to oxidative stress biomarkers in blood and follicular fluid, to embryo quality and pregnancy success? Oxid Med Cell Longev 2019:1092415. https://doi.org/10.1155/2019/1092415
Nagy RA, van Montfoort APA, Groen H, Homminga I, Andrei D, Mistry RH et al (2019) Anti-oxidative function of follicular fluid HDL and outcomes of modified natural cycle-IVF. Sci Rep 9(1):12817. https://doi.org/10.1038/s41598-019-49091-3
Jain SK, Micinski D (2013) Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun 437(1):7–11. https://doi.org/10.1016/j.bbrc.2013.06.004
Tavakoli F, Namakin K, Zardast M (2016) Vitamin D supplementation and high-density lipoprotein cholesterol: a study in healthy school children. Iran J Pediatr 26(4):e3311. https://doi.org/10.5812/ijp.3311
Ansari MGA, Sabico S, Clerici M, Khattak MNK, Wani K, Al-Musharaf S et al (2020) Vitamin D supplementation is associated with increased glutathione peroxidase-1 levels in arab adults with prediabetes. Antioxidants (Basel). https://doi.org/10.3390/antiox9020118
Francis EC, Hinkle SN, Song Y, Rawal S, Donnelly SR, Zhu Y et al (2018) Longitudinal maternal vitamin D status during pregnancy is associated with neonatal anthropometric measures. Nutrients. https://doi.org/10.3390/nu10111631
Leffelaar ER, Vrijkotte TG, van Eijsden M (2010) Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr 104(1):108–117. https://doi.org/10.1017/S000711451000022X
Gernand AD, Simhan HN, Klebanoff MA, Bodnar LM (2013) Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a U.S. multicenter cohort study. J Clin Endocrinol Metab 98(1):398–404. https://doi.org/10.1210/jc.2012-3275
Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM (2013) Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ 346:f1169. https://doi.org/10.1136/bmj.f1169
Morley R, Carlin JB, Pasco JA, Wark JD (2006) Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 91(3):906–912. https://doi.org/10.1210/jc.2005-1479
Eggemoen ÅR, Jenum AK, Mdala I, Knutsen KV, Lagerløv P, Sletner L (2017) Vitamin D levels during pregnancy and associations with birth weight and body composition of the newborn: a longitudinal multiethnic population-based study. Br J Nutr 117(7):985–993. https://doi.org/10.1017/S000711451700068X
Wei SQ, Qi HP, Luo ZC, Fraser WD (2013) Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 26(9):889–899. https://doi.org/10.3109/14767058.2013.765849
Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K (2012) Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod 27(11):3321–3327. https://doi.org/10.1093/humrep/des280
The level of vitamin D in follicular fluid and ovarian reserve in an in vitro fertilization program: A pilot study (all levels <25 ng) - May 2022
Science Progress 105(2) https://doi.org/10.1177/00368504221103782
Ji Yeon Han, Sung Woo Kim, Hoon Kim, ...
Background
The level of vitamin D in follicular fluid (FF) according to the ovarian reserve has never been investigated, and the effect of FF vitamin D on the outcome of assisted reproductive technology (ART) remains controversial. The aim of this study is to evaluate the association between FF vitamin D levels and baseline anti-Müllerian hormone (AMH) / ART outcomes.
Methods
Forty-seven patients who underwent controlled ovarian stimulation at the fertility clinic of an academic tertiary care center were enrolled for a prospective observational study. FF was collected from the first aspirated leading follicle of each ovary and assayed by an enzyme-linked immunosorbent assay. Multivariable linear regression analysis was used to assess the association between baseline AMH and FF vitamin D levels with adjustment for basal FSH and serum vitamin D levels.
Results
Both the AMH and serum vitamin D were significant predictors for FF vitamin D. The estimated marginal mean of FF vitamin D level was higher in women with decreased ovarian reserve (DOR) than those with normal ovarian reserve (24.1 ± 2.1 vs. 18.8 ± 1.4 ng/ml, p = 0.048). However, FF vitamin D did not demonstrate any significant associations with cycle outcomes , including fertilization rate and the number and proportion of good embryos at day three.
Conclusion
We observed significantly higher FF vitamin D levels in women with DOR. However, FF vitamin D did not demonstrate any significant associations with the outcome of ART. A larger prospective study is needed to investigate the effect of FF vitamin D on the clinical pregnancy rate and live birth rate.
📄 Download the PDF from VitaminDWiki
FF Vit D levels corrlated with pregnancy test, oocyte count - (did not consider VDR) - July 2022
Follicular fluid 25-hydroxyvitamin D levels determine fertility outcome in patients with polycystic ovary syndrome
Taiwan J Obstet Gynecol. 2022 Jul;61(4):620-625. doi: 10.1016/j.tjog.2022.03.041
Ramazan Ozyurt 1, Cemil Karakus 2
Objective: To determine the possible relationship between follicular fluid 25-hydroxyvitamin D [25(OH)D] levels and fertility outcome of women who underwent IVF/ICSI with the diagnosis of lean polycystic ovary syndrome.
Materials and methods: Thirty patients who were diagnosed with PCOS according to the Rotterdam criteria and decided on IVF/ICSI were included in the study. Thirty patients who were scheduled for IVF/ICSI for reasons other than PCOS and matched in terms of age and BMI were taken as the control group (non-PCOS). According to BMI values, patients in both PCOS and non-PCOS groups were lean. Women in both groups were aged 21-35 years with a normal BMI (18.5-24.9 kg/m2) and first IVF/ICSI attempt. Both groups of patients were followed up using the antagonist protocol. Vit D levels were measured in serum and follicular fluid (FF) samples taken on the day of oocyte collection. The correlation between FF vit D levels, the number of total oocytes, MII oocytes and 2 PN zygotes, HOMA-IR, hormonal and demographic parameters, clinical pregnancy rate (CPR) , live birth rate (LBR), and miscarriage rate were evaluated.
Results: At the time of oocyte retrieval women with PCOS had similar serum Vitamin D compared to non-PCOS women (21.8 (12.6-24.8) ng/ml vs 22.3 (11.5-25.1) ng/ml, p < 0.54). In FF, assessed on the day of oocyte retrieval, the concentration of Vitamin D was similar in women with PCOS when compared to non-PCOS women (11.2 (9.2-14.4) ng/ml vs 13.3 (11.1-17.4) ng/ml, p < 0.06). For both groups, Vitamin D levels were lower in FF compared to serum vit D. A positive correlation was found between serum and FF Vitamin D concentrations in the full cohort. A positive and significant correlation was found between FF-vit D levels and the number of total oocyte (r = 0.344, p < 0.04) and MII oocyte (r = 0.404, p < 0.02) in the PCOS group. The number of total oocyte, MII oocyte and 2 PN zygotes of the PCOS group were significantly higher than the non-PCOS group. Positive pregnancy test rate, clinical pregnancy and live birth rates were similar in both groups. The miscarriage rates in the non-PCOS group were significantly higher than in the PCOS group.
A positive and significant correlation was also found between FF vit D levels and positive pregnancy test (r = 0.566, p < 0.03) and CPR (r = 0.605, p < 0.02) in PCOS group.
There was no correlation between FF-vit D levels and live birth and miscarriage rates in neither the PCOS nor the non-PCOS group.
Conclusions: Both serum and FF 25-hydroxyvitamin D level of women with PCOS at the time of oocyte retrieval are similar to non-PCOS controls. While FF 25-hydroxyvitamin D levels correlate with total and MII oocyte counts, positive pregnancy test and CPR, it does not correlate with miscarriage and live birth rates.
📄 Download the PDF from VitaminDWiki
Follicular Fluid and follicles

A follicle is a small sac of fluid in the ovaries that contains a developing egg.
One egg per follicle
Women begin puberty with about 300,000 to 400,000 eggs.
Each monthly menstrual cycle a number of follicles, each containing an egg, are selected to grow and mature
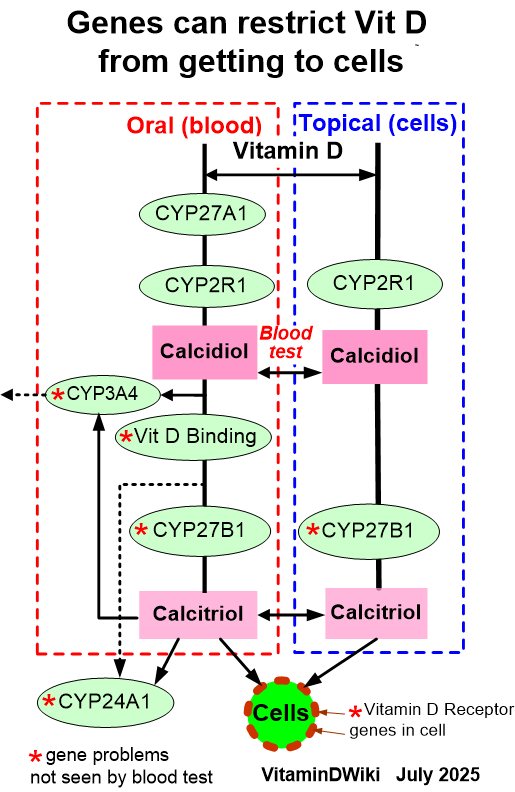
Agreed: Local level of Vitamin D is more important than blood/serum level Vitamin D
At least 5 genes can restrict the level of vitamin D which actually gets to the cell
However, It is both expensive and difficult to measure cell-level of Vitamin D
Many studies have found that high levels of vitamin D (50-70 ng) in the blood greatly increase the cellular Vitamin D
2 groups keep increasing vitamin D dose until the PTH is reduced (Vitamin actually getting to cells
Examples:
Vitamin D is needed for human fertility – goal is 50 ng – Sept 2018
Vitamin D level in eye not associated with level in blood – Jan 2020
Different Vitamin D levels to stop restrictions due to poor or disease-altered genes
{include}
VitaminDWiki - Vitamin D greatly improves Fertility
{include}
VitaminDWiki - Genetics chart (Receptor is the most important and easily changed)
The risk of 44 diseases at least double with poor Vitamin D Receptor
VitaminDWiki - Vitamin D Receptor activation can be increased in 13+ ways:
Omega-3, Magnesium, Zinc, Quercetin, non-daily Vit D, Curcumin, intense exercise, Ginger, Essential oils, etc Note: The founder of VitaminDWiki uses 10 of the 13 known VDR activators
VitaminDWiki - studies in both categories Fertility and Vitamin D Receptor
This list is automatically updated
{category}
Title change made July 2022 caused the visitor count to reset.
There have actually been visitors to this page since it was originally made
