- Vitamin D and viral infections:Infectious diseases, autoimmune diseases, and cancers
- Introduction
- Infectious diseases
- Autoimmune diseases
- Viruses and cancer
- Conclusion
- VitaminDWiki – COVID-19 treated by Vitamin D - studies, reports, videos
- VitaminDWiki – Overview Influenza and vitamin D
- VitaminDWiki – Respiratory viral infection (RSV) and low vitamin D - many studies
- VitaminDWiki – Infections and low vitamin D - many studies
- VitaminDWiki – Dengue Fever decimated by Vitamin D - many studies
- VitaminDWiki – Epstein-Barr Virus probably causes Long-COVID, CFS, and MS - many studies
- VitaminDWiki – Cancer category contains:
- 11+ VitaminDWiki Virus pages have CANCER in the title
Vitamin D and viral infections:Infectious diseases, autoimmune diseases, and cancers
Advances in Food and Nutrition Research ISSN 1043-4526, https://doi.org/10.1016/bs.afnr.2023.12.007
William B. Grant: Sunlight, Nutrition and Health Research Center, San Francisco, USA wbgrant at infionline.net
Abstract
Viruses can cause many human diseases. Three types of human diseases caused by viruses are discussed in this chapter: infectious diseases, autoimmune diseases, and cancers. The infectious diseases included in this chapter include three respiratory tract diseases: influenza, COVID-19, and respiratory syncytial virus. In addition, the mosquito-borne dengue virus diseases are discussed. Vitamin D can reduce risk, severity, and mortality of the respiratory tract diseases and possibly for dengue virus. Many autoimmune diseases are initiated by the body's reaction to a viral infection. The protective role of vitamin D in Epstein-Barr virus-related diseases such as multiple sclerosis is discussed. There are a few cancers linked to viral infections. Such cancers include cervical cancer, head and neck cancers, Hodgkin's and non-Hodgkin's lymphoma, and liver cancer. Vitamin D plays an important role in reducing risk of cancer incidence and mortality, although not as strongly for viral-linked cancers as for other types of cancer.
Download the PDF from VitaminDWiki
Introduction
Viruses are the cause of many human diseases including respiratory tract diseases (Da Silva et al., 2013, Kusel, de Klerk, et al., 2006, Pavia, 2011, Vos, Bruyndonckx, et al., 2021), autoimmune diseases (Hussein & Rahal, 2019, Sundaresan, Shirafkan, Ripperger, & Rattay, 2023), and several types of cancer (Moore & Chang, 2010; Zur Hausen, 1991). Vitamin D plays an important role in reducing risk of many of the diseases linked to viruses. This chapter covers the role of solar ultraviolet radiation-B (UVB) and vitamin D in reducing risk of incidence, severity, and mortality for these diseases with an emphasis on the epidemiology and some discussion of the mechanisms whereby vitamin D reduces risk. The discussion regarding infectious diseases, such as respiratory tract infections, is extensive, while the discussions regarding autoimmune diseases and cancers are brief. The extent of the coverage for these three disease categories is in line with the extent of research by the author. The author has done considerable research on the roles of solar UVB and vitamin D on risk, severity, and mortality of influenza (Grant & Giovannucci, 2009) and COVID-19 (Grant, Lahore, et al., 2020) as well as cancer incidence and mortality rates (Grant & Garland, 2006; Munoz & Grant, 2022). He has also done research on some of the autoimmune diseases including autism (Grant & Cannell, 2013) and multiple sclerosis (MS) (Grant, 2008a, 2008b). Thus, the author has the background required to evaluate the research findings regarding vitamin D and viral infections as risk factors for various diseases.
Infectious diseases are discussed first. The ones included are viruses that affect the respiratory tract, influenza, SARS-CoV-2, and respiratory syncytial virus (RSV), and two other viruses, Epstein-Barr virus (EBV) and dengue virus (DNV).
Infectious diseases
Influenza
John Cannell and colleagues published a seminal article regarding the role of vitamin D in reducing risk of influenza in 2006 (Cannell, Vieth, et al., 2006). The first evidence for vitamin D and influenza was the figure published by Hope-Simpson in 1981 showing that type A influenza outbreaks peak around January in the northern hemisphere temperate latitudes and July in southern hemisphere temperate latitudes (Hope-Simpson, 1981). However, inspection of the seasonal variation in serum 25-hydroxyvitamin D [25(OH) D] concentrations, shows that the minimum 25(OH)D concentrations in Great Britain (Hypponen & Power, 2007) and the US (Kroll, Bi, et al., 2015) occur in February or March.
Subsequent studies noted that the seasonality of influenza outbreaks is regulated by meteorological conditions. An animal model study reported in 2007 noted that influenza virus transmission is dependent on relative humidity and temperature (Lowen, Mubareka, Steel, & Palese, 2007). An observational study reported in 2009 found that absolute humidity modulates influenza survival, transmission, and seasonality, with transmission strongly inversely correlated with absolute humidity (Shaman & Kohn, 2009). A paper from 2012 reported that humidity levels below 6 g of water vapor per kg of air were significantly associated with influenza mortality rates (Barreca & Shimshack, 2012).
An article published in 2019 provided plausible mechanisms to explain the effects of temperature and humidity on survival, transmission, and incidence of the influenza virus (Marr, Tang, Van, & Lakdawala, 2019). The primary effect was related to aerosol droplet size. At low relative humidity, aerosol diameters become small due to evaporation of water. A 10-gm diameter aerosol stays suspended for 8 min while a 1.9-g.m diameter aerosol stays suspended for 3 h. In addition, smaller diameter aerosols are able to be deposited in the alveolar regions of the lungs rather than in the upper respiratory tract. The small-diameter aerosols are much more likely to result in infection in the lungs than in the upper respiratory tract. The effect of higher temperature may also be to inactivate proteins and nucleic acid (Woese, 1960).
An observational study of influenza virus activity in Nordic countries during 2010-2018 gave further support for the roles of ambient temperature and humidity in affecting virus activity (Ianevski, Zusinaite, et al., 2019), but also added the effect of solar ultraviolet (UV) dose. Influenza activity was pronounced between weeks 45 and 9. During that period, the UV is only UVA with the result that no vitamin D is produced during that time. The UV effect was intermediate between that for temperature and humidity. The likely mechanism explaining the UV effect was liberation of nitric oxide (NO) from subcutaneous nitrate stores (Grant & Boucher, 2022, Weller, Mahrhofer, Davis, & Gorman, 2021).
Serum 25(OH)D concentrations and seasonal variations are insufficient evidence that vitamin D affects risk of influenza in a causal manner. The best way to determine causality regarding vitamin D is through randomized controlled trials (RCTs). The first successful vitamin D RCT regarding influenza was conducted with school children in Japan (Urashima, Segawa, et al., 2010). Schoolchildren aged 6-15 y, with or without underlying diseases, were eligible and asked to participate in the study by the pediatricians in charge of the outpatient clinics. The accrual period was from 1 November 2008 to 15 December 2008. Four hundred thirty children were invited to participate in the study and 334 were followed until the end of the study. Those in the vitamin D treatment group took 1200 IU/day vitamin D3 while those in the control group took placebos. Influenza A occurred in 18 of 167 (10.8%) children in the vitamin D3 group compared with 31 of 167 (18.6%) children in the placebo group [relative risk (RR), 0.58 (95% CI: 0.34, 0.99; p = 0.04)]. The reduction in influenza A was more prominent in children who had not been previously taking vitamin D supplements [RR: 0.36 (95% CI: 0.17, 0.79; P = 0.006)] and who started nursery school after age 3 y [RR: 0.36 (95% CI: 0.17, 0.78; p = 0.005)]. However, 39 of 167 in the vitamin D treatment group and 28 of 167 in the placebo group developed influenza B; the relative risk for vitamin D treatment vs. placebo was 1.39 (95% CI, 0.90-2.15).
In addition, a subsequent vitamin D RCT conducted by the same author during the 2009 H1N1 influenza pandemic did not find that 2000 IU/day vitamin D reduced risk of influenza (Urashima, Mezawa, Noya, & Camargo, 2014). A rapid influenza diagnostic test showing positive for influenza A occurred in 20/148 (14%) of the students in the vitamin D3 group and 12/99 (12%) in the placebo group (risk ratio [RR] = 1.11[95% CI, 0.57-2.18]). A similar finding was made for influenza-like illness. As mentioned by the author at a symposium in 2015, many of the children had been vaccinated against influenza in that period. In addition, it is very likely that vitamin D is less likely to reduce risk of pandemic influenza incidence than that of epidemic influenza. For example, there was no evidence that vitamin D reduced risk of the 1918-1919 pandemic influenza incidence although it did reduce risk of mortality from pneumonia (Grant & Giovannucci, 2009).
A carefully designed and conducted vitamin D RCT on seasonal influenza A was conducted on infants in China (Zhou, Du, et al., 2018). A total of 400 infants 3-12 months old who attended a hospital in Jinhua, China were enrolled between October 2015 and May 2016. Half were randomly assigned a 400 IU/day vitamin D3 supplement (low-dose group) for 4 months while another half was assigned 1200 IU/day (high-dose group). Baseline 25(OH)D concentrations were 17 ± 2 ng/mL; achieved 25(OH)D concentrations after 2 or 4 months were 25 ± 4 ng/mL in the high-dose group and 17 ± 2 ng/mL in the low-dose group. Some infants were dropped from the study for various reasons. During the study, 78 of 168 in the low-dose group developed influenza A, while 43 of the 164 in the high-dose group developed influenza. The risk ratio for influenza A for high- vs. low-dose was 0.56 (95% CI, 0.42-0.77, p = 0.0002). In addition, the status of the infants was carefully monitored every four hours after initiation of influenza A until body temperature returned to normal, then once every 24 h. As shown in Figure 2 in that article, the percentage of patients with fever dropped to 10% in 48 h for the high-dose group and 80 h in the low-dose group. Figure 3 shows that the percentage of patients with cough dropped to 10% in four days for the high-dose group and 7 days for the low-dose group. Figure 4 shows that the percentage of patients with wheezing dropped to 10% in 3 days for the high-dose group and 4.5 days for the low-dose group. Thus, higher-dose vitamin D therapy reduced the duration of elevated temperature, cough, and wheezing in this study.
Generally considered better than a single vitamin D RCT is a metaanalysis of several vitamin D RCTs. A meta-analysis of 15 vitamin D RCTs regarding incidence of influenza reported from 2012 to 2019 was published in 2022 (Zhu, Zhu, et al., 2021). In total, there were 265 events for 2472 participants in the vitamin D arms and 327 events for the 2387 participants in the control arms, yielding a risk ratio (RR) = 0.78 (95% CI, 0.42-0.77), i.e., a 22% reduction. However, inspection of the studies included in the meta-analysis finds that several types of influenza and related illnesses were included: influenza A, influenza B, influenza A + influenza B, and influenza-like illness. Since three of the studies separated influenza A and influenza B, it is worthwhile to look at those results separate from the others. Table 1 gives the details regarding four of these trials.
The Vietnam study participants had a mean baseline 25(OH)D concentration of 27 ng/mL in the vitamin D treatment arm and 26 ng/mL in the control arm. These concentrations are likely high enough so that the participants already had some protection against influenza. However, the fact that so many participants developed influenza suggests that the effect of vitamin D in reducing risk of influenza is weak.
Two vitamin D supplementation studies not included in the metaanalysis show that serum 25(OH)D concentrations of participants should have been much lower. In the first RCT, based on 208 postmenopausal African-American women in New York with half taking 800 IU/day for the first two years, followed by 2000 IU/day vitamin D3 in the third year, and half taking a placebo (Aloia and Li-Ng, 2007), the baseline 25(OH)D concentration was 19 ± 8 ng/mL. The outcome was based on colds and influenza symptoms. Thirty participants taking the placebo had a cold/ influenza, 8 taking 800 IU/day, and 1 taking 2000 IU/day.
Table 1 Details of three RCTs regarding vitamin D supplementation and incidence of influenza.
See PDF
In the second vitamin D RCT by the same group consisting of162 participants, with 65% of the participants Caucasian, ~80% female, age ~59 ±13 years, were enrolled in a 12-month study (Li-Ng, Aloia, et al., 2009). Half were randomized to receive 2000 IU/day and half a placebo. The baseline 25(OH)D concentration was 25 ± 11 ng/mL. The achieved 25(OH)D concentration was near 36 ± 9 ng/mL. Fifty-six percent of the vitamin D treatment group had been vaccinated against influenza, and 64% of the placebo group had been vaccinated against influenza. There was no difference in the incidence of upper respiratory tract infections (URIs) between the vitamin D and placebo groups (48 URIs vs. 50 URIs, respectively, p = 0.57). There was no difference in the duration or severity of URI symptoms between the vitamin D and placebo groups [5.4 ± 4.8 days vs. 5.3 ± 3.1 days, respectively, p = 0.86].
A review of the evidence regarding vitamin D and influenza was published in August 2018 (Gruber-Bzura, 2018). The author reviewed the evidence from many types of studies regarding influenza and respiratory tract infections (RTIs) including observational studies with respect to season and serum 25(OH)D concentration and vitamin D RCTs. This review also looked at the effects of vitamin D on immunogenicity of influenza vaccines. The overall conclusion was that vitamin D does have effects on innate and adaptive immune responses that should be useful for reducing risk and severity of influenza, but that studies through June 2018 had not proven a causal benefit of vitamin D against influenza. However, that review did not include the apparently strong study from China, which was published in August 2018 (Zhou, Du, et al., 2018).
It is important to note that it is calcitriol that is the vitamin D metabolite that activates the innate immune system, mainly through intracrine production from circulating 25(OH)D (Gombart, 2009; White, 2012). Circulating calcitriol plays a very small role compared to its intracellular effects. Macrophages and mitochondria have both the enzyme CYP27B1 and the vitamin D receptor (VDR), enabling them to convert 25(OH)D to calcitriol and then affect gene expression through the VDR. Calcitriol, through the VDR, is also involved in regulation of genes encoding antimicrobial peptides. A 2019 comprehensive review of how innate leukocytes (white blood cells) react to influenza virus infection was published (Lamichhane & Samarasinghe, 2019). Table 2 outlines some of the roles of vitamin D for the innate and adaptive immune system responses to influenza.
Pandemic influenza
Pandemic influenza arises when the influenza virus type is one that is much more virulent than epidemic virus types. The virus type in the 1918-1919 influenza pandemic was H1N1 (Francis, McNeil, et al., 2019). That type was also responsible for the 1977 and 2009 influenza pandemics (Francis, McNeil, et al., 2019). There is no evidence that solar UVB, a proxy for vitamin D in 1918-1919, had any effect on development of influenza in that period. However, a geological ecological study conducted with respect to case-fatality rates for 13 U.S. communities (Britten, 1932) found that mortality rates were reduced by about 50% for communities in the southern states (Grant & Giovannucci, 2009). In addition, reductions in rates of pneumonia as a complication of influenza were reduced by approximately one-third. The proposed explanation for these findings included that vitamin D reduced the production of pro-inflammatory cytokines, which could lead to a “cytokine storm” that could damage the epithelial layers of the lungs. Also, induction of human cathelicidin and defensins could reduce the viability of bacteria that could lead to pneumonia. More recent articles have outlined the mechanisms involved in progressing from infection to disease. Figure 1 in a 2021 article outlined the immune pathways triggered by influenza-virus infection (Zhu, Zhu, et al., 2021). The virus infects the alveolar or airway epithelial cells. Viral RNA in the cytoplasm recognized by toll-like receptors and Nod-like receptors leads to production of interferons (IFNs). Type I IFNs interfere with viral replication, but if it is overproduced, it can form the cytokine storm. Type II and type III IFNs have different roles to play. People under the age of 5 years and over the age of 65 years are more likely to develop cytokine storms. Women, especially those of childbearing age, are more likely to die from influenza than are men, attributed to difference in estrogen and testosterone concentrations. Obesity also increases risk of serious outcomes from influenza due to increased inflammation and other effects.
Adaptive immunity may play a more important role in protecting people from pandemic influenza than the innate immune system. An analysis of age-specific influenza mortality rates found that mortality rates for those <5 and 20-45 years were much higher during the 1918 influenza pandemic in the U.S. compared to the mortality rates for epidemic influenza in 1915 (Ma, Dushoff, & Earn, 2011). Similar results were found in comparing pandemic influenza (H2N2) mortality rates in Canada for 1957 with epidemic influenza mortality rates for 1968, this time with significantly higher rates for ages 12-40 years. The antigenic history hypothesis is that an H2 subtype similar to the 1957 strain circulated prior to 1918, thereby providing some protection for people born prior to 1918 (>40 years of age in 1957). The authors noted that the 2009 H1N1 pandemic displayed elevated mortality among those born after 1957, indicating that antigenic imprinting may be the major cause of protection (Chowell, Bertozzi, et al., 2009). This is indicative of an effect of the adaptive immune system, likely affected by serum 25(OH)D concentration.
Table 2 Role of vitamin D for the innate and adaptive immune system against influenza.
See PDF
SARS-CoV-2/COVID-19
Near the onset of the COVID-19 pandemic, my colleagues and I suggested that vitamin D could reduce the risk of SARS-CoV-2 infection and progression to COVID-19 (Grant, Lahore, et al., 2020). We based our proposal on what we thought we knew about the role of vitamin D in reducing risk of influenza and other respiratory tract infections at that time.
Vitamin D helps maintain tight junctions, gap junctions, and adherens junctions (e.g., by E-cadherin) (Schwalfenberg, 2011). Several articles discussed how viruses disturb junction integrity, increasing infection by the virus and other microorganisms (Chen, Wu, et al., 2020; Kast, McFarlane, et al., 2017; Rossi, Fanous, & Colin, 2020). Disturbed junction integrity seems to be an important reason why pneumonia develops for those with influenza and COVID-19 (Grant, Lahore, et al., 2020). As discussed already, I hypothesized that solar UVB, though production of vitamin D, reduced risk of death from pandemic influenza followed by pneumonia during the 1918-1919 influenza pandemic (Grant & Giovannucci, 2009).
Another mechanism of protection from infection is the induction of antimicrobial peptides such as defensins and cathelicidins (Gombart, Borregaard, & Koeffler, 2005; Wang, Nestel, et al., 2004). Those host- derived peptides kill the invading pathogens by perturbing their cell membranes and can neutralize the biological activities of endotoxins (Agier, Efenberger, & Brzezinska-Blaszczyk, 2015).
The innate immune system generates both pro-inflammatory and antiinflammatory cytokines in response to viral and bacterial infections, as observed in COVID-19 patients (Huang, Wang, et al., 2020). Vitamin D can reduce the production of pro-inflammatory T helper cell type 1 (Th1) cytokines, such as tumor necrosis factor-a and interferon-y (Sharifi, Vahedi, Nedjat, Rafiei, & Hosseinzadeh-Attar, 2019).
Vitamin D is a modulator of adaptive immunity; 1,25(OH)2D3 (calcitriol) suppresses responses mediated by Th1, by primarily repressing production of inflammatory cytokines interleukin-2 and interferon-gamma (INFy) (Lemire, Adams, et al., 1985). Additionally, 1,25(OH)2D3 promotes cytokine production by the T helper type 2 (Th2) cells, which helps enhance the indirect suppression of proinflammatory Th1 cells (Cantorna, Snyder, Lin, & Yang, 2015). Furthermore, 1,25(OH)2D3 promotes induction of the T regulatory cells, thereby inhibiting inflammatory processes (Jeffery, Burke, et al., 2009).
Vitamin D supplementation also enhances the expression of genes related to antioxidation (glutathione reductase and glutamate-cysteine ligase modifier subunit) (Lei, Zhang, Cheng, & Lee, 2017). The increased glutathione production spares the use of ascorbic acid (vitamin C), which has antimicrobial activities (Marik, Kory, Varon, Iglesias, & Meduri, 2021), and has been proposed and studied to prevent and treat COVID-19 (Shahbaz, Fatima, et al., 2022).
Later, my colleagues and I examined the role of additional mechanisms whereby vitamin D reduces risk of COVID-19 (Mercola, Grant, & Wagner, 2020). The understanding of the role of anti-microbial peptides (AMPs) had been expanded. As shown in Figure 1 in that review one of the functions of AMPs is chemotaxis, the movement of cells in response to a chemical stimulus, specifically macrophages, mast cells, monocytes, and neutrophils. Other effects of vitamin D include activation of the innate immune system, effects on angiogenesis, antiendotoxin activity, and opsonization. Opsonization is the molecular mechanism whereby pathogenic molecules, microbes, or apoptotic cells (antigenic substances) are connected to antibodies, complement, or other proteins to attach to the cell surface receptors on phagocytes and NK cells.
Cell cultures of human alveolar type II cells with vitamin D have shown that the SARS-CoV-2 virus interacts with the Angiotensin-converting enzyme 2 (ACE2) receptor expressed on the surface of lung epithelial cells. Once the virus binds to the ACE2 receptor, it reduces its activity and, in turn, promotes ACE1 activity, forming more angiotensin II, which increases the severity of COVID-19 (Bavishi, Maddox, & Messerli, 2020; Rolf,2020). The vitamin D metabolite calcitriol increases concentrations of ACE2 in the lungs of experimental animals (Xu, Yang, et al., 2017). The additional ACE2 expressed as a consequence of vitamin D supplementation might reduce lung injury (Aygun, 2020) because it can promote binding of the virus to the pulmonary epithelium. Various studies have shown that vitamin D has a crucial role in protecting against acute lung injury and acute respiratory distress syndrome (ARDS) in experimental models (Rhodes, Subramanian, Laird, Griffin, & Kenny, 2021; Yan, Xiao, & Lin, 2020).
Vitamin D also reduces damage from renin-angiotensin system (RAS)- mediated bradykinin (BK) storm in the progression of COVID-19 (Garvin, Alvarez, et al., 2020). BK is a potent part of the vasopressor system that induces hypotension and vasodilation. BK is degraded by ACE and enhanced by the angiotensin produced by ACE2. The authors suggested that vitamin D could reduce the risk of the BK storm through several mechanisms including regulation of RAS. Renin is the enzyme that catalyzes the first step in the activation pathway of angiotensinogen by cleaving angiotensinogen to form angiotensin I, which is then converted to angiotensin II by angiotensin I converting enzyme. In the COVID samples, analyzed renin levels were increased 380-fold compared to controls. Vitamin D is a negative endocrine RAS modulator and inhibits renin expression and generation (Ross, Manson, et al., 2011). It appears likely that vitamin D deficiency amelioration would limit the COVID-19 BK storm. However, further investigation is needed to evaluate the role of vitamin D in this context. Also, vitamin D deficiency contributes to acute respiratory distress syndrome, for which rates increased with age and with chronic disease comorbidity (Dancer, Parekh, et al., 2015).
One of the important findings regarding COVID-19 is that those below the age of 18 years develop COVID-19 with reduced severity (Williams, Howard-Jones, et al., 2020). SARS-CoV-2 infection only progresses to COVID-19, the disease, if the immune system is unable to limit the proliferation of the virus and ramps up the production of pro-inflammatory cytokines. In the U.S. during the period before COVID-19 vaccines were available (June to August, 2020), confirmed COVID-19 cases were somewhat elevated for those in the 20-49 years age groups, between 300 and 550 cases/ 100,000, dropping to around 200/100,000 for those aged 70-79 (Boehmer, Kompaniyets, et al., 2021). Interestingly rates for those >80 years went from ~400/100,000 in May to ~260 in August. On the other hand, mortality rate rises dramatically with increasing age. An ecological study involving 16 countries with a total population of 2.4 billion people as of April 12, 2020, found that the mortality rate for those aged 55-64 years was 8.1 (95% CI, 7.7-8.5) times that for those aged <54 years, and 62 (95% CI, 60-64) times higher for those >65 years (Yanez, Weiss, Romand, & Treggiari, 2020).
The reason why mortality rates are high in the elderly is that the immune system declines. A 2020 article outlined that the possible pathophysiolical mechanism of cytokine storms in elderly adults with COVID-19 may be due to “Inflame-aging” (Meftahi, Jangravi, Sahraei, & Bahari, 2020).
A study conducted in Greece found significantly higher NK cell count for 7 COVID-19 patients in the ICU with serum 25(OH) concentrations between 20 and 30 ng/mL (107 [95% CI, 89-170]) than for the 32 with serum 25(OH)D concentration <20 ng/mL (88 [95% CI, 53-125, p = 0.048]) (Vassiliou, Jahaj, et al., 2021).
Serum 25(OH)D concentration is lowered when one has an acute inflammatory illness that raises body temperature (Smolders, van den Ouweland, Geven, Pickkers, & Kox, 2021). The innate immune system causes a large number of pro-inflammatory cytokines to be released to fight the infection, thereby raising body temperature and lowering serum 25(OH)D concentrations as vitamin D moderates the cytokine balance between pro- and anti-inflammatory cytokines. Consequently, measuring serum 25(OH)D concentration at time of admission to a hospital is not very useful for determining the relationship between serum 25(OH)D concentration and risk of COVID-19. However, it is useful in determining the probability of survival. This was demonstrated in an observational study conducted in the United Arab Emirates. This observational study used data for 464 participants who tested positive for SARS-CoV-2 at one of the main hospitals in Abu Dhabi and Dubai. Serum 25(OH)D was measured at the time of hospital admission. In the fully-adjusted model, the OR for severe COVID-19 with low serum 25(OH)D was 1.76 (1.19, 2.61) 0.005, while the OR for mortality for 25(OH)D <12 ng/mL was 2.58 (1.01, 6.62) 0.048. While age was also a significant factor for severity and mortality, it raised risk by about 7%.
A 2020 study determined the correlation between serum 25(OH)D concentration and severe acute SARS-CoV-2 positivity rates (Kaufman, Niles, Kroll, Bi, & Holick, 2020). The study was based on over 190,000 patients in the US who had SARS-CoV-2 results performed between midMarch through mid-June 2020 by a national testing company and had serum 25(OH)D concentration measurements performed in the preceding 12 months. The 25(OH)D concentration data were seasonally adjusted. Residential zip code was used to determine latitude of the patients and race/ethnicity data were also determined. The mean SARS-CoV-2 positivity rate was 9.3% (95% C.I. 9.2-9.5%) and the mean seasonally adjusted 25(OH)D was 31.7 (SD 11.7) ng/mL. The most interesting result is in Figure 2B in that study. It shows the SARS-CoV-2 positive rate vs. serum 25(OH)D concentration for different race/ethnicity. The positive rates for <20 ng/mL and >50 ng/mL are: 9% and 5% for white non-Hispanics, 16% and 8% for Hispanics, and 19% and 11% for Black non-Hispanics. The rates are reduced by 45-50% for high vs. low 25(OH)D concentrations for each race/ethnicity. The higher rates for Hispanics and Black non-Hispanics are most likely due to the fact that they generally have lower socioeconomic status and, thus, are less able to socially isolate at home and work than white people. This article provided strong evidence that vitamin D status significantly affected SARS-CoV-2 positivity rate.
Two articles reported observational studies of COVID-19 risk with respect to vitamin D supplementation. The first one was based on adults living in Barcelona-Central Catalonia (Oristrell, Oliva, et al., 2022). Both cholecalciferol (vitamin D3) and calcifediol [25(OH)D] are mostly available only by prescription and records of who was prescribed either form were available. Patients on cholecalciferol treatment achieving 25OHD concentrations >30 ng/mL had lower risk of SARS-CoV-2 infection, lower risk of severe COVID-19 and lower COVID-19 mortality than unsupplemented 25OHD-deficient patients (56/9474 [0.6%] vs 96/7616 [1.3%]; HR 0.66 [CI 95% 0.46-0.93], p = 0.02). Patients on calcifediol treatment achieving serum 25OHD concentrations > 30 ng/mL also had lower risk of SARS-CoV2 infection, lower risk of severe COVID-19, and lower COVID-19 mortality compared to 25OHD-deficient patients not receiving vitamin D supplements (88/16,276 [0.5%] vs. 96/7616 [1.3%]; HR 0.56 [CI 95% 0.42-0.76], p < 0.001).
The second study was an observational study conducted on patients of the US Veterans Administration Health Care System who received supplementation with vitamin D2 or vitamin D3 between January 1, 2019 and December 31, 2020 and during the pandemic (March 1, 2020 to December 31, 2020), and had at least one serum 25(OH)D concentration measurement between January 1, 2019 and December 31, 2020 (Gibbons, Norton, et al., 2022). They found that vitamin D2 and D3 supplementation was associated with reductions in COVID-19 infection of 28% and 20%, respectively [(D3 HR = 0.80, [[95% CI 0.77, 0.83]), D2 HR = 0.72, [[95% CI 0.65, 0.79]]. Mortality within 30-days of COVID-19 infection was similarly 33% lower with Vitamin D3 and 25% lower with D2 (D3 HR = 0.67, [95% CI 0.59, 0.75]; D2 HR = 0.75, [95% CI 0.55, 1.04]). They also found that after controlling for serum 25(OH)D concentrations, veterans receiving higher dosages of vitamin D obtained greater benefits from supplementation than veterans receiving lower dosages. Veterans with presupplementation serum 25(OH)D concentrations between 0 and 19 ng/mL exhibited the largest decrease in COVID-19 infection following supplementation. Black veterans received greater associated COVID-19 risk reductions with supplementation than White veterans since Black people generally have lower serum 25(OH)D concentrations compared to white people (Ames, Grant, & Willett, 2021).
COVID-19 develops rapidly. Thus, it is necessary to use vitamin D and other natural compounds as early as possible if not prior to infection. The important effects of vitamin D are to reduce the viability of the SARS- CoV-2 virus, to reduce the risk of the cytokine storm, to maintain lung epithelial lining integrity, and to reduce concomitant bacterial infection in order to reduce risk of ensuing pneumonia. Thus, the earlier the better. Also, serum 25(OH)D concentration should be raised rapidly. While cal- cifediol raises serum 25(OH)D within hours, the oral administration of even high doses of vitamin D takes three to five days to raise serum 25(OH) D concentrations. This delay is due to its less efficient absorption than calcifediol and the need for vitamin D to undergo 25-hydroxylation in the liver, a rate-limiting step (Wimalawansa, 2022).
Physicians in Cordoba, Spain have been using calcifediol to treat COVID-19 patients. The first report was in spring of 2021 (Entrenas Castillo, Entrenas Costa, et al., 2020). The participants were 76 consecutive patients hospitalized with COVID-19 infection, with a clinical picture of acute respiratory infection (ARI), confirmed by a radiographic pattern of viral pneumonia, and by a positive and severe SARS-CoV-2 PCR. All hospitalized patients received the best available standard care, (per hospital protocol). Eligible patients were allocated at a 2 calcifediol:1 no calcifediol ratio through electronic randomization on the day of admission to take oral calcifediol (0.532 mg), or not. Patients in the calcifediol treatment group continued with oral calcifediol (0.266 mg) on day 3 and 7, and then weekly until discharge or ICU admission. Of 50 patients treated with calcifediol, one required admission to the ICU (2%), while of 26 untreated patients, 13 required admission (50%) p < 0.001. The multivariate risk estimate OR for patients in ICU with calcifediol treatment vs. without calcifediol treatment ICU (adjusting by Hypertension and T2DM: 0.03 [95%CI: 0.003-0.25]). Of the patients treated with calcifediol, none died, and all were discharged, without complications. The 13 patients not treated with calcifediol, who were not admitted to the ICU, were discharged. Of the 13 patients admitted to the ICU, two died and the remaining 11 were discharged.
An observational study regarding mortality with respect to serum 25(OH)D concentration for 551 consecutive COVID-19 patients in a hospital in Mexico City was reported in 2022 (Vanegas-Cedillo, Bello- Chavolla, et al., 2022). The mean age of the patients was 53 ± 14 years, mortality was near 20%, mean BMI was 30 ± 6 kg/m2. Many additional parameters were given. The statistically significant ones comparing those with serum 25(OH)D concentration greater or less than 20 ng/mL at time of the hospital visit were male sex, type 2 diabetes mellitus, oxygen saturation, HbAlc, D-dimer, fibrinogen, number of comorbidities, and both epicardial and subthoracic fat. Some of these parameters are risk factors, while others are biomarkers of the disease effects. In the fully adjusted model, the HR for mortality with respect to serum 25(OH)D was 0.96 (95% CI, 0.94-0.99, p = 0.006). The HR for mortality with respect to oxygen saturation was 0.98 (0.96-0.99. p = 0.001). There was a plot of HR for mortality vs. 25(OH)D concentration with 1.0 set at 20 ng/mL. For 25(OH)D concentration near zero, the HR was 2.1.
A systematic review and meta-analysis was conducted to evaluate the efficacy of vitamin D supplementation in COVID-19 for patients admitted to a hospital (Sirbu, Sabin, Bocsan, Vesa, & Buzoianu, 2023). Thirteen RCTs were included. Based on data from six RCTs, vitamin D supplementation reduced length of stay by 1.5 (95% CI, -3.1 to 0.02) days, p = 0.05. Based on data from eight RCTs, vitamin D supplementation reduced admission to the ICU by 37% (RR = 0.63 [95% CI, 0.41-0.99], p = 0.04). However, vitamin D supplementation did not reduce risk of death. Based on data from eight RCTs, RR = 0.93 (95% CI, 0.62-1.52, p = 0.78). The most likely reason for the poor results is that those admitted to the hospital had advanced COVID-19 so that it was too late for the most important roles of vitamin D regarding COVID-19. A second reason is that most vitamin D RCTs included used vitamin D3, which takes several days to increase serum 25(OH)D concentrations (Wimalawansa, 2023).
A meta-analysis of nine observational studies regarding vitamin D deficiency and COVID-19 in-hospital mortality before mRNA vaccinations were used was reported in a 2021 review (Ebrahimzadeh, Mohseni, et al., 2021). The OR for mortality with vitamin D deficiency was 2.11 (95% CI, 1.03-4.32, p = 0.003). As previously discussed, having COVID- 19 lowers serum 25(OH)D concentration. Thus, this result is useful regarding risk or mortality related to serum 25(OH)D concentration after admission to a hospital, but not regarding vitamin D status prior to COVID-19.
Although there is strong scientific evidence that vitamin D could reduce risk and severity of COVID-19, the general public was not informed. One reason was that in order to obtain an emergency authorization for the mRNA vaccines against SARS-CoV-2, it had to be asserted that there were no other effective ways to reduce risk. Under section 564 of the Federal Food, Drug, and Cosmetic Act, when the Secretary of the US Health and Human Services declares that an Emergency Use Authorization (2023) is appropriate, the FDA may authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by chemical, biological, radiological, and nuclear threat agents when certain criteria are met, including there are no adequate, approved, and available alternatives (2023). Thus, the FDA had to assert that vitamin D, Ivermectin, and many other over-the-counter compounds could not be used to prevent COVID-19. In addition, the FDA used the “Disinformation Playbook” to prevent the spread of information and use of vitamin D and other compounds. The Disinformation Playbook’s use by the pharmaceutical industry to discourage use of vitamin D was outlined in 2018 (Grant 2018). Among other things, the news media and much of social media was prohibited from discussing vitamin D and other inexpensive repurposed drugs and neutraceuticals in a positive way regarding COVID-19.
Respiratory syncytial virus
RSV is responsible for a large number of acute lower respiratory tract infections (ALRI) in young children. A 2010 review estimated that in 2005 an estimated 34 (95% CI, 19-46) million new episodes of RSV-associated ALRI occurred worldwide in children younger than 5 years (22% of ALRI episodes). In 2005, an estimated 66,000—199,000 children died from RSV-associated ALRI, primarily in developing countries.
RSV is also an important ALRI for adults, especially the elderly. A meta-analysis of RSV by age group for various continents was published in a 2020 review (Tin Tin Htar, Yerramalla, Moisi, & Swerdlow, 2020). In the US and Canada, the proportion of ARI that were from RSV was 4% (95% CI, 2-6%) for those <50 years, 3% (95% CI, 1-5%) for adults, and 7% (95% CI, 5-9%) for those >50 years. For Europe, proportion of ARI that were from RSV was 9% (95% CI, 4-17%) for those <50 years, 7% (95% CI, 4-11%) for adults, and 10% (95% CI, 5-16%) for those >50 years.
An analysis of influenza-like illness (ILL) and acute bronchitis (largely due to RSV) incidence rates in the UK for those >65 years between 1967/ 68 and 2005/06 found that up until 1983/84, acute bronchitis incidence rates were about twice those of ILL. However, after that period, acute bronchitis rates increased by about a factor of three while ILL rates decreased by about 50% (Elliot & Fleming, 2008). The reductions in ILL were attributed to widespread use of influenza vaccinations.
An observational study in France examined the propensity for bacterial co-infection with RSV in hospitalized elderly patients (Godefroy, Giraud- Gatineau, et al., 2020). A total of 12,144 hospitalized patients with ARI were screened for RSV, resulting in finding 701 with RSV. Of the 701, 85 had a bacterial co-infection. The in-hospital mortality rate for those with bacterial co-infection was 2.94 (95% CI, 1.30-6.60). Death was often from bacteria-associated pneumonia.
There are several approaches to evaluating whether vitamin D plays an important role in reducing risk of RSV. They include seasonality of the disease, whether alleles of genes involved in the vitamin D metabolic pathway are associated with risk of RSV, whether serum 25(OH)D concentrations are lower for those with RSV than controls, whether vitamin D supplementation reduces risk of RSV, and how the RSV virus interacts with the innate and adaptive immune system. Each of these approaches has strengths and limitations. Nonetheless, when considered in toto, they may help reach a conclusion.
Since solar UVB exposure is an important source of vitamin D, it is logical to think that higher solar UVB exposure would reduce the risk of RSV if vitamin D plays an important role. A 2007 article reported an analysis of seasonal variations in RSV activity with respect to meteorological conditions at five locations in the US plus four international locations (Yusuf, Piedimonte, et al., 2007). In temperate climates, RSV activity was maximal in winter, correlating with lower temperatures. In locations with high temperature and humidity, RSV activity had reduced seasonality. The authors suggested that RSV had greater stability in the aerosols there. Nonetheless, solar UVB dose was estimated to explain 13% of RSV activity in Miami, FL and 5% in Buffalo, NY.
A 2007 article reported that severe bronchiolitis, RSV, and pneumonia rates among young children in Hawaii varied according to ethnicity (Yorita, Holman, et al., 2007). I noted that RSV rates they reported were highest in winter and correlated with skin pigmentation with lowest rates for whites, then Japanese, Chinese, other Asian, Filipino, Hawaiian, other Pacific Islander, and black (Grant, 2018c). Since temperature and humidity effects should be the same regardless of skin pigmentation, I proposed that differences in serum 25(OH)D concentrations related might explain their findings.
Many subsequent articles have reported seasonality of RSV. Table 3 gives the finding from several studies. For the years prior to the COVID-19 pandemic, the RSV season was generally 20-30 weeks long, with the peak near the period of lowest solar zenith angle. However, during the COVID-19 period, the RSV season was shifted considerably. The reason may relate to measures taken to reduce risk of COVID-19 such as lock downs and mask wearing (Olsen, Azziz-Baumgartner, et al., 2020). However, as discussed regarding influenza, peak activity in winter does not necessarily mean that vitamin D was the cause. Seasonal variations of RSV in other countries are given in a 2018 review (Obando-Pacheco, Justicia-Grande, et al., 2018).
Studies of vitamin D-related gene polymorphisms with respect to RSV activity may be able to provide stronger evidence for an effect of vitamin D. Findings on this topic are given in Table 4. While these three studies did find significant correlations, each study had evaluated a number of singlenucleotide polymorphisms (SNPs), 384 SNPs in 220 candidate genes (Janssen, Bont, et al., 2007) and found very few that were significant. Thus, these could have been chance findings.
One of the important effects of the innate immune response to viral infections is increasing human cathelicidin (LL-37) concentrations in the serum and other fluids in the lungs. A 2013 laboratory study found that LL-37 had effective antiviral activity against RSV in vitro (Currie, Findlay, et al., 2013). LL-37 prevented virus-induced cell death in epithelial cultures, significantly inhibited the production of new infectious particles and diminished the spread of infection, with antiviral effects directed both against the viral particles and the epithelial cells. That work was extended in 2016 (Currie, Gwyer, Findlay, et al., 2016). LL-37 mediates an antiviral effect on RSV by inducing direct damage to the viral envelope, disrupting viral particles and decreasing virus binding to, and infection of, human epithelial cells in vitro. In addition, exogenously applied LL-37 is protective against RSV-mediated disease in vivo, in a murine model of pulmonary RSV infection, demonstrating maximal efficacy when applied concomitantly with the virus. Furthermore, endogenous murine cathe- licidin, induced by infection, has a fundamental role in protection against disease in vivo postinfection with RSV. Finally, higher nasal levels of LL-37 are associated with protection in a healthy human adult RSV infection model.
Table 3 Periods of high RSV activity in the US in different years.
See PDF
Table 4 Findings regarding vitamin D-related gene polymorphisms with respect to RSV activity.
See PDF
A 2011-2014 observational study found that LL-37 was inversely correlated with likelihood of requiring intensive care with infant’s bronchiolitis due to RSV or rhinovirus (Mansbach, Hasegawa, et al., 2017). A total of 1005 infants with mean age 3 months were enrolled in 17 centers. In multivariable models, infants with LL-37 levels < 60 ng/mL compared with >60 ng/mL, were more likely to have RSV (e.g., aOR, 2.6 [lowest quartile]; p < 0.001 [all quartiles]). LL-37 levels are increased at higher 25(OH)D concentrations.
A laboratory study using RSV-infected human bronchial epithelial cells with disrupted epithelial barrier disruption demonstrated that calcitriol triggered protein kinase A activity in these cells, thereby restoring the barrier function of these cells (Gao, Raduka, & Rezaee, 2023). Additional information regarding the mechanisms by which vitamin D reduces risk of RSV are in a 2015 review (Greiller & Martineau, 2015).
The relation between serum 25(OH)D concentration and severity of RSV at time of hospitalization is another way to determine the effect of vitamin D on RSV. A 17-center prospective cohort study of 1016 US infants <12 months of age hospitalized with bronchiolitis examined the severity of the disease with respect to serum 25(OH)D concentration (Vo, Koppel, et al., 2018). In multivariable models, infants with total 25(OH)D < 20 ng/mL had higher risk of requiring intensive care (aOR 1.72, 95% CI 1.12-2.64) and longer length of stay (adjusted rate ratio 1.39, 95% CI 1.17-1.65) compared with infants with total 25(OH)D > 30 ng/mL. While the authors of this study noted that having an acute inflammatory illness was not found to affect serum 25(OH)D concentrations, that idea was subsequently overturned (Smolders, van den Ouweland, et al., 2021). Thus, this study does not provide information regarding the role of serum 25(OH)D concentration on risk of RSV but does provide information on the effect of serum 25(OH)D concentration after hospitalization on severity.
RSV is a common disease among infants and cord blood serum 25(OH) D concentration has been found inversely correlated with risk and severity of RSV. A study in The Netherlands included 156 neonates (Belderbos, Houben, et al., 2011). Eighteen of them developed RSV lower respiratory tract infection. The mean 25(OH)D concentration for those who developed RSV was 26 ng/mL compared with 34 ng/mL for those who did not (p = 0.009). Neonates born with cord blood 25(OH)D concentrations <20 ng/mL compared to those with >30 ng/mL had an adjusted relative risk of RSV in the first year of life of 6.2 (95%-CI: 1.6-24.9, p = 0.01). Cord blood 25(OH)D concentration was strongly correlated with maternal serum 25(OH)D concentrations.
An observational study in Finland examined the association of serum 25(OH)D concentration with probability of viral co-infection for hospitalized wheezing children (Jartti, Ruuskanen, Mansbach, Vuorinen, & Camargo, 2010). Infections were RSV or rhinovirus or both. The unadjusted probability of co-infection decreased from 70% for 25(OH)D concentration of 5 ng/mL to 45% at 14 ng/mL, 35% at 40 ng/mL, and 10% at 100 ng/mL. However, there were few cases with 25(OH)D above 50 ng/mL. What this means is that any effective clinical trial would have to enroll participants with 25(OH)D concentration below 8 ng/mL and not give any of those in the control arm any vitamin D, such as was done in Mongolia. That one included 141 children with mean age 10 ± 1 years and mean 25(OH)D concentration 7 ± 2 ng/mL given 300 IU/day vitamin D3 in milk and 105 similar controls (Camargo, Ganmaa, et al., 2012). Those in the vitamin D arm achieved 19 ± 3 ng/mL and had fewer ARIs than the control arm during the three months of the trial ([0.45 ± 0.86] vs. [0.80 ± 0.95], p = 0.047).
The ideal way to determine whether vitamin D reduces risk of RSV is through an RCT. A survey of the journal literature indicates that the nearest that RCTs come to addressing this question is through examining the effect of vitamin D supplementation on acute respiratory infection. A 2016 review of the prevalence of RSV in Western countries involved 98 studies. RSV was associated with 12-63% of all ARIs and 19-81% of all viral ARIs causing hospitalizations in children (Bont, Checchia, et al., 2016). There are a number of vitamin D RCTs regarding incidence or ARIs. A 2017 meta-analysis found that vitamin D supplementation reduced risk of ARIs. 25 eligible RCTs (total 11,321 participants, aged 0 to 95 years) were identified (Martineau, Jolliffe, et al., 2017). Individual participant data were obtained for 10,933 participants. Vitamin D supplementation reduced the risk of acute respiratory tract infection among all participants (aOR = 0.88 [95% CI, 0.81-0.96; P]). In subgroup analysis, protective effects were seen in those receiving daily or weekly vitamin D without additional bolus doses (aOR 0.81 [0.72-0.91]) but not in those receiving one or more bolus doses (aOR = 0.97 [95% CI, 0.86 to 1.10]). Among those receiving daily or weekly vitamin D, protective effects were stronger in those with baseline 25(OH)D concentrations <10 ng/mL (aOR = 0.30 [95% CI, 0.17-0.53]) than in those with baseline 25(OH)D concentrations >10 ng/mL (aOR = 0.75 [95% CI, 0.60-0.95]). Vitamin D did not influence the proportion of participants experiencing at least one serious adverse event (aOR = 0.98 [95% CI, 0.80-1.20]). (Martineau, Jolliffe, et al., 2017). An updated version of this meta-analysis including 46 RCTs with 75,541 participants became available. Data for the primary outcome were obtained for 48,488 (98-1%) of 49,419 participants (aged 0-95 years) in 43 studies (Jolliffe, Greenberg, et al., 2019). Similar results were obtained.
A 2021 review concluded its review of vitamin D and RSV by stating it seems that vitamin D has the capability to modulate the inflammatory response to RSV infection and prevent severe disease without attenuating the immune system ability to clear RSV, which partly justifies the results of some of the aforementioned clinical investigations (Vaghari-Tabari, Mohammadzadeh, et al., 2023).
An observational study conducted in Argentina examined the effect of serum 25(OH)D concentration on risk of life-threatening RSV disease (LTD) (Ferolla, Yfran, et al., 2022). A total of 125 previously healthy infants <12 months of age who needed intensive care and ventilator care with a first RSV episode were enrolled during 2017-2019. Of those, 22 developed LTD. The mean 25(OH)D concentration for those who developed LTD was 18 ng/mL (IQR, 15-30 ng/mL) vs. 32 ng/mL (IQR, 24-42 ng/mL). 59% of infants with LTD had vitamin D deficiency compared with 12% in those with better outcome. Multivariable regression analysis confirmed vitamin D deficiency as a risk factor (OR, 11.83 [95% confidence interval, 3.89-35.9; P < .001]). It should be noted that acute inflammatory diseases lower serum 25(OH)D concentrations (Smolders, van den Ouweland, et al., 2021). Thus, this study should be considered making findings regarding severity and survival in a hospital situation rather than regarding risk of RSV incidence.
Dengue virus
Infection with any of the Dengue virus (DENV) serotypes may be asymptomatic in the majority of cases or may result in a wide spectrum of clinical symptoms, ranging from a mild flu-like syndrome (known as dengue fever [DF]) to the most severe forms of the disease, which are characterized by coagulopathy, increased vascular fragility, and permeability (dengue hemorrhagic fever [DHF]). The latter may progress to hypovolemic shock (dengue shock syndrome [DSS]) (Martina, Koraka, & Osterhaus, 2009). Figure 1 in that review shows the proposed model for the pathogenesis ofDF, DHF, and DSS.
Globally from 1990 to 2019, dengue incident cases, deaths, and disability- adjusted life years (DALYs) gradually increased. Those under 5 years of age, once accounting for the largest portion of deaths and DALYs in 1990, were eclipsed by those who were 15-49 years old in 2019. South-East Asia and South Asia had most of the dengue incident cases, deaths and DALYs, with DALYs rising in other tropical regions. Global land-ocean temperature index and air passenger travel metrics were found to be remarkably positively correlated with dengue burden (Yang, Quam, Zhang, & Sang, 2021).
A 2012 laboratory study found that calcitriol reduced the number of infected cells and reduced the levels of proinflammatory cytokines (Puerta- Guardo, Medina, et al., 2012).
Macrophages represent important cell targets for DENV replication and consequently, they are key drivers of dengue disease. In this study they evaluated the effect of vitamin D3 on the differentiation of monocyte- derived macrophages (MDM) and their susceptibility and cytokine response to DENV. The data demonstrates that MDM differentiated in the presence of vitamin D3 (D3-MDM) restrict DENV infection and moderate the classical inflammatory cytokine response. Mechanistically, vitamin D3-driven differentiation led to reduced surface expression of C-type lectins including the mannose receptor (MR, CD206) that is known to act as primary receptor for DENV attachment on macrophages and to trigger of immune signaling. Consequently, DENV bound less efficiently to vitamin D3-differentiated macrophages, leading to lower infection (Tripkovic, Wilson, et al., 2017).
Clinical manifestations of dengue disease rely on complex interactions between DENV and host factors that drive altered immune responses, including excessive inflammation (Arboleda, Fernandez, & Urcuqui- Inchima, 2019). This group recently established that vitamin D can modulate DENV-induced cytokine responses and restrict infection in human macrophages. Cytokine responses are finely regulated by several homeostatic mechanisms, including microRNAs (miRNAs) that can rapidly target specific genes involved in the control of immune signaling pathways. In further laboratory studies, this group used a qPCR miRNA array to profile immune-related miRNAs induced by DENV infection in human monocyte-derived macrophages (MDM), differentiated in absence or presence of vitamin D3 (D3-MDM). They found several miRNAs differentially expressed in both MDM and D3-MDM upon DENV infection. The study suggests a key role of vitamin D3 in the control of inflammatory cytokine responses during DENV infection of human macrophages via the Toll-like receptor-4 (TLR4)/nuclear factor kappa-B (NF-KB)/miR-155-5p/SOCS-1 axis.
A study aimed to assess the impact of oral vitamin D supplementation on DENV-2 infection, TLR expression, and both pro- and anti-inflammatory cytokine production in monocyte-derived dendritic cells (MDDCs) (Martinez- Moreno, Hernandez, & Urcuqui-Inchima, 2020). Twenty healthy donors were randomly divided into two groups and received either 1000 or 4000 IU/day of vitamin D for 10 days. During pre- and post-vitamin D supplementation, peripheral blood samples were taken to obtain MDDCs, which were challenged with DENV-2. They found that MDDCs from donors who received 4000 IU/ day of vitamin D were less susceptible to DENV-2 infection than MDDCs from donors who received 1000 IU/day of vitamin D. Moreover, these cells showed decreased mRNA expression of TLR3, 7, and 9; downregulation of interleukins IL-12/IL-8 production; and increased IL-10 secretion in response to DENV-2 infection. These findings support a possible role of vitamin D in improving the innate immune response against DENV.
A study involving healthy human volunteers was conducted to evaluate the effect of vitamin D3 supplementation the extent and kinetics of innate immune responses of DENV-2 infected monocytes differentiated into macrophages in the presence (D3-MDMs) or absence of vitamin D3 (MDMs) (Castillo, Giraldo, et al., 2021). They found that D3-MDMs expressed lower levels of retinoic acid inducible gene-I (RIG-I), TLR3, and TLR7, as well as higher levels of suppressor of cytokine signaling 1 (SOCS-1) in response to DENV-2 infection. D3-MDMs produced lower levels of reactive oxygen species, related to a lower expression of TLR9. Moreover, although vitamin D3 treatment did not modulate either the expression of IFN-a or IFN-P, higher expression of protein kinase R (PKR) and 2'-5'-oligoadenylate synthetase 1 (OAS1) mRNA were found in D3-MDMs. Importantly, the observed effects were independent of reduced infection, highlighting the intrinsic differences between D3- MDMs and MDMs. Taken together, these results suggest that differentiation of MDMs in the presence of vitamin D3 modulates innate immunity responses to DENV-2 infection.
A study assessed the effect of LL-37 on DENV-2-induced responses in human monocyte-derived macrophages (MDMs) (Castillo, Giraldo, Smit, Rodenhuis-Zybert, & Urcuqui-Inchima, 2022). It showed that simultaneous exposure of exogenous LL-37 and DENV-2 resulted in reduced replication of the virus in MDMs, while the addition ofLL-37 post exposure to DENV-2 did not. They also demonstrated that vitamin D3 increased LL-37 concentrations.
Another study of vitamin D3, miRNAs, and DENV was reported in 2023 (Castillo & Urcuqui-Inchima, 2023). It found that vitamin D3 supplementation into human volunteers inhibited several inflammatory of miRNAs, led to decreased production of TNF-a and TLR9 expression, while increased the expression of SOCS-1, IFN-P, and OAS1, without affecting DENV replication. By contrast, over-expression of miR-182-5p, miR-130a-3p, miR-125b- 5p, and miR-155-5p significantly decreased DENV-2 infection rates and also DENV-2 replication in MDMs.
Two observational studies found that higher serum 25(OH)D concentrations were associated with increased risk of progression from DF to DHF/DSS. The first study was conducted in Columbia (Villamor, Villar, Lozano, Herrera, & Herran, 2017). It involved 110 cases that progressed to DHF/DSS and 235 controls that did not progress to those diseases from DF. The significant differences between cases and controls were that cases were older (15% <15 years vs. 30% <15 years), and the episode was in March/April, the months with highest solar UVB intensity (36% vs. 15%). The two factors associated with serum 25(OH)D concentration >30 nmol/L compared to <20 nmol/L were male sex (59% vs. 11%) and age <15 years (35% vs. 0%). The multivariable-adjusted ORs for progression to DHF/DSS compared to >30 ng/mL were 0.44 (95% CI, 0.22-0.99) for 20-<30 ng/mL and 0.13 (95% CI, 0.02-1.05), p = 0.003. The authors speculated that lower vitamin D binding protein (VDBP) in cases might explain the findings by affecting bioavailable 25(OH)D.
The second observational study was conducted in Pakistan (Ghafoor, Siddiqi, et al., 2023). The mean age of the patients was 29 ± 17 years. Serum 25(OH)D concentrations were inversely correlated with platelet count and hematocrit. Serum 25(OH)D concentrations for those with severe DF were 38 ± 32 ng/mL while concentrations for those with nonsevere DF were 16 ± 3 ng/mL.
An observational study conducted in Sri Lanka with children found that DHF/DSS were associated with low, not high serum 25(OH)D concentrations in comparison with healthy controls. The mean age of the participants was 9 ± 3 years. The adjusted OR for <20 ng/mL vs. >20 ng/mL was 3.65 (95% CI, 1.46-9.19). The authors of this study stated that the difference between their results and those of Villamor, Villar, et al. (2017) may be explained by differences in case definitions, DENV serotypes, age of the participants, and disease severity.
Autoimmune diseases
The 2023 review by Sundaresan, Shirafkan, et al. (2023) lists the viruses that cause four important autoimmune diseases. For rheumatoid arthritis, they are Human T-cell leukaemia virus type 1 (HTLV-1), a retrovirus, and EBV. For multiple sclerosis (MS), they are herpes simplex virus (HSV), EBV, human herpesvirus, varicella-Zoser virus, and human endogenous retroviruses. For systemic lupus erathematosus (SLE), it is EBV. For diabetes mellitus type 1 (T1DM), they are coxsackle B4, cytomegalovirus, rotovirus, and rubella. Table 1 in that review also gives the target cells of self-peptides, immune cells or cytokines involved, and pathomechanisms. The 2019 review by Hussein and Rahal (Hussein & Rahal, 2019) includes a few more viruses and autoimmune diseases including human T-lymphotropic virus 1, measles virus, enterovirus serotype CV, hepatitis C virus and other members of the flaviviridae family, and human parvovirus B19.
A 2017 review examined the role of season regarding development, severity, and progression of autoimmune diseases (Watad, Azrielant, et al., 2017). Late winter and early spring, a time when serum 25(OH)D concentrations are lowest, are associated with increased disease activity for MS, non-cutaneous flares of SLE, psoriasis, and RA. In addition, higher rotovirus infections in winter preceded T1DM onset.
The role of vitamin D in reducing risk of autoimmune diseases was covered in another chapter in this book (Brown, Marchwicka, & Marcinkowska, 2024). The autoimmune diseases discussed include type 1 diabetes mellitus, inflammatory bowel disease, rheumatoid arthritis, and MS. Only MS, infectious mononucleosis, and EBV will be discussed in this chapter.
Epstein-Barr virus and multiple sclerosis
The EBV is a double-stranded DNA virus belonging to the Herpes family. It is the primary cause of infectious mononucleosis (IM) (Fugl & Andersen, 2019). It is also implicated in many other diseases including lymphopro- liferative disorders, head and neck cancer, breast cancer, SLE, chronic fatigue syndrome, thyroid disorders, rheumatoid arthritis, and MS (Fugl & Andersen, 2019). The discussion regarding EBV will start with IM and then review what is known about EVB and MS.
IM is more frequently reported in winter/spring than in the fall. A study in northern Scotland found peak rates in February-March and lowest rates in July-October, with rates ~38% higher in winter than in summer (Visser, Milne, et al., 2014). A study conducted in Norway found IM rates higher in spring than in the fall (Lossius, Riise, et al., 2014). It also found a significant correlation between IM and MS.
There is strong evidence that EBV is an important risk factor for MS. A 2010 review noted that EBV is a risk factor for IM and IM increases the risk of developing MS 2 to 3-fold (Ascherio & Munger, 2010). Also, the risk of MS is very low in individuals who are EBV negative but increases several fold following EBV infection.
Very strong support for EBV being the viral cause of MS comes from a 2022 analysis of active duty personnel in the US military (Bjornevik, Cortese, et al., 2022). A total of 955 personnel developed MS between 1993 and 2013. Pre-diagnosis serum was analyzed for viruses. Risk of MS increased by a factor of 32 after EVB infection but was not increased after infection with other viruses.
MS prevalence has a very pronounced latitudinal distribution, rising from very low below 40° N to (Kurtzke, 2000). A 2022 international registry study found that the severity of MS did not change below 40°N and increased in a linear fashion to 57° N (Vitkova, Diouf, et al., 2022). Latitude is generally considered an index of UV dose. A 2021 review based on global burden of disease data and the World Health Organization along with the UV index (UVI) from the Tropospheric Emission Monitoring Internet Service website evaluated the effect of UVI on incidence, prevalence, DALYs, and mortality of MS (Maghbooloi, Sahraian, et al., 2021). MS incidence rates were increased 3.5 (95% CI, 2.5-4.5) for low UVI, 1.7 (95% CI 1.4-2.0) for moderate UVI, and 1.5 (0.9-2.1) for high UVI.
Note that the UVI is predominantly related to UVA (315-400 nm), not UVB (290-315 nm). Thus, the UVI index is weakly related to vitamin D production. In addition, it is impossible to produce vitamin D from UVB for about 6 months of the year at the latitude of Boston (42.6° N) (Webb, Kline, & Holick, 1988). This leaves open the possibility that solar UVA as well as UVB have some influence on the incidence of MS.
A 2021 review evaluated the effect of both latitude and serum 25(OH) D on MS severity (Ostkamp, Salmen, et al., 2021). It used data from 946 treatment-naive MS patients in the NationMD cohort in Germany (~48-54° N) and 990 natalizumab-treated MS patients in France (~44-50° N) in the BIONAT cohort. By using data for severity of MS using Gd-enhancing lesions, they were able to show very significant inverse correlations with 25(OH)D concentrations and direct correlations with latitude. This study provides good evidence that sunlight exposure has beneficial effects on patients with established MS.
An analysis of data from two US cohorts, Nurses’ Health Study and Nurses’ Health Study II was made to determine the effects of latitude, ambient temperature, and UV radiation on incidence of MS (Ohaegbulam, Swalih, Patel, Smith, & Perrin, 2020). Both decreasing latitude and increasing temperature were associated with increased incidence of MS. However, no significant association was found for UV radiation. Latitudes in the US range from ~25° to ~45° N. As the studies in Europe found effects for UV above 40° N, the finding regarding UV in the US is not surprising.
Another way to determine the effect of vitamin D on risk of MS is through use of Mendelian randomization (MR) studies. MR studies of the effects of vitamin D use values for the effect on circulating serum 25(OH)D for alleles of various factors in the vitamin D pathway. These values are found from genome-wide association studies of participants with the same ethnic background of those in the MR study. Use of these values then assign participants according to their genetically-determined 25(OH)D concentration. When sufficient numbers of participants are included, the effects of factors that affect concentrations such as vitamin D supplementation, diet, and solar UVB exposure are averaged out. MR studies are considered nearly equivalent to RCTs in determining the effects of vitamin D on health outcomes (Hypponen, Vimaleswaran, & Zhou, 2022). A 2021 review performed a MR study of risk factors for MS (Yuan, Xiong, & Larsson, 2021). It used data for 14,498 MS cases and 24,091 controls of European ancestry. Childhood and adulthood BMI were directly correlated with MS while physical activity and serum 25(OH)D concentration were inversely correlated with MS. The weighted-median method gave an OR for vitamin D = 0.83 (95% CI, 0.72-0.95). A second MR study investigated the effect of serum 25(OH)D and BMI on risk and relapse hazard in MS (Vandebergh, Dubois, & Goris, 2022). It used 4 distinct genome-wide association studies for serum 25(OH)D in up to 416,247 individuals. A 1-SD increase in genetically predicted naturallog transformed 25OHD concentrations decreased odds of MS up to 28% (95% CI: 12-40%) and decreased hazard for a relapse occurring up to 43% (95% CI: 15-61%).
Two 2022 reviews outlined the roles of vitamin D and MS, one in reducing risk (Gombash, Lee, Sawdai, & Lovett-Racke, 2022), the other regarding the mechanisms of vitamin D for immunomodulation in MS along with therapeutic implications (Galoppin, Kari, et al., 2022). (Could expand on these studies).
A 2019 review compared results regarding vitamin D and disease activity in MS (Smolders, Torkildsen, Camu, & Holmoy, 2019). It noted that while observational studies find that a 10 ng/mL increase in serum 25(OH)D is associated with 14-34% reduced relapse risk and 15-50% reduced risk of new lesions on magnetic resonance imaging, two large RCTs failed to find a benefit of vitamin D supplementation on disease activity. The authors suggested problems with both observational studies and the RCTs may explain the discrepancy between the two approaches. However it is now clear that the observational study results are correct. It is also clear that most vitamin D RCTs have failed to find beneficial health effects (Grant, Boucher, Al, & Pilz, 2022). The primary reason is that they have been designed based on guidelines for pharmaceutical drugs, not nutrients. Heaney outlined the guidelines for nutrient RCTs in 2014 (Heaney, 2014). The main guidelines applied to vitamin D include that serum 25(OH)D concentration be measured prior to entry and those with low concentrations be selected for participation, vitamin D doses have to be large enough so that serum 25(OH)D is optimized for the outcome of interest, achieved serum 25(OH)D concentrations have to be measured and used in the analysis of the outcomes. Very few if any vitamin D RCTs have followed these guidelines.
Viruses and cancer
Viral infections cause a number of types of cancer. These include EBV for Burkitt’s lymphoma, B-lymphomas in immunosuppressed individuals, and nasopharyngeal cancer. Hepatitis B virus (HBV) increases risk of hepatocellular carcinoma. Human papillomavirus (HPV) for anal, cervical, oral, penile, perianal, and vulvar cancer (Acharya, Dalia, et al., 2021; Zur Hausen, 1991).
Non-Hodgkin’s lymphoma (NHL) has many subtypes including Burkitt’s lymphoma and B-cell lymphoma (Thandra, Barsouk, et al., 2021). Various viruses have been linked to risk of NHL such as EBV for Burkitt’s lymphoma and hepatitis C for B-cell lymphoma. Other factors are also associated with risk of NHL such as obesity, tobacco smoking, and alcohol consumption. Thus, the relative contributions of viruses to risk can be variable. However, specific viruses can be very important risk factors in some locations such as EBV for children for Burkitt’s lymphoma in equatorial Africa, Brazil and Egypt (Thandra, Barsouk, et al., 2021) and chronic HBV for B-cell lymphoma in Taiwan (Su, Liu, et al., 2019).
Solar UVB doses have been found significantly inversely correlated with NHL incidence and mortality rates in the US in geographical ecological studies (Boscoe & Schymura, 2006; Grant & Garland, 2006). In both studies, the associations remained significant after other riskmodifying factors were included in the analysis such as alcohol consumption and smoking prevalence.
A prospective observational study conducted in Iowa, USA examined prognosis in NHL with respect to serum 25(OH)D concentration (Drake, Maurer, et al., 2010). The mean age of the 983 participants at time of enrollment was 62 years (range, 19-94 years). 44% had serum 25(OH)D concentrations <25 ng/mL within 120 days of diagnosis. The mean follow-up time was 35 months. There were 193 deaths including 168 from NHL. After adjusting for known prognostic factors and treatment, patients with 25(OH)D <25 ng/mL with diffuse large B-cell lymphoma had inferior event-free survival (hazard ratio [HR], 1.41 [95% CI, 0.98 to 2.04]) and overall survival (HR, 1.99 [95% CI, 1.27 to 3.13]); patients with 25(OH)D <25 ng/mL with T-cell lymphoma also had inferior event-free survival (HR, 1.94; 95% CI, 1.04 to 3.61) and overall survival (HR, 2.38 [95% CI, 1.04 to 5.41]). There were no associations with event-free survival for the other NHL subtypes.
The association of 25(OH)D concentration with HPV cervicovaginal infection for women in the US was determined using data from the US National Health and Nutrition Examination Survey 2003-2006 data (Shim, Perez, Symanski, & Nyitray, 2016). Data were used for 2353 sexually-active women. After adjustment for age, race/ethnicity, and marital status, the odds of high-risk HPV infection were increased per each 10 ng/mL decrease in serum 25(OH)D concentration (aOR, 1.14 [ 95% CI, 1.02-1.27]). Several studies have found an inverse relationship between the incidence of cervical neoplasia and serum 25(OH)D concentrations (Avila, Noriega-Mejia, et al., 2023). The narrative review by Avila, Noriega-Mejia, et al. (2023) updates the current evidence supporting the notion that the vitamin D endocrine system has a preventive role on cervical cancer, mainly in the early phases of the disease, acting at the level of suppressing cell proliferation, promoting apoptosis, modulating inflammatory responses, and probably favoring the clearance of HPV-dependent cervical lesions.
HPV is also a risk factor for head and neck squamous cell carcinoma (HNSCC). An observational study in Germany examined the serum 25(OH)D concentrations for 231 HNSCC patients an 232 healthy controls (Bochen, Balensiefer, et al., 2018). Mean serum 25(OH)D concentrations were significantly lower in HNSCC patients than in controls (~11 ng/mL vs. ~25 ng/mL, p < 0.001). However, HNSCC patients who were HPV-negative had lower mean 25(OH)D concentrations than those who were HPV-positive (~9 ng/mL vs. ~15 ng/mL, p = 0.01). However, of the 35 HPV positive patients, HPV-positive with low 25(OH)D low, n = 18; HPV-positive, high 25(OH)D, n = 17. Thus, with the low numbers, it is not clear what the role of vitamin D is for HPV. Further discussion of the role of vitamin D in HNSCC is found in a 2023 review (Starska-Kowarska, 2023).
Conclusion
Viruses are responsible for many human diseases with respiratory tract infections being ofgreatest concern. There is good evidence that vitamin D can reduce the risk of incidence of, severity of, and death from influenza, COVID-19, and RSV. Serum 25(OH)D concentrations in the range of 30-60 ng/mL are suggested for prevention. For treatment, serum 25(OH) D concentrations should be raised rapidly starting as soon as possible with either high-dose vitamin D3 or, preferably, calcifediol. For DF, there is reasonable evidence that vitamin D reduces risk. However, there have been reports that treating DF with high-dose vitamin D may actually increase mortality rates. For cancer, higher 25(OH)D concentrations were found to reduce risk of two types of NHL: diffuse large B-cell lymphoma and T-cell lymphoma. Solar UVB doses have also been found inversely correlated with incidence and mortality rates of NHL.
References
- A charya, P., Dalia, T., Ranka, S., Sethi, P., Oni, O. A., Safarova, M. S., ... Barua, R. S. (2021). The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. Journal of the Endocrine Society, 5(10) bvab124.
- Agier, J., Efenberger, M., & Brzezinska-Blaszczyk, E. (2015). Cathelicidin impact on inflammatory cells. Central European Journal of Immunology, 40(2), 225—235.
- Aglipay, M., Birken, C. S., Parkin, P. C., Loeb, M. B., Thorpe, K., Chen, Y., ... Collaboration, T. A. K. (2017). Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA: The Journal of the American Medical Association, 318(3), 245—254.
- Ahmed, F. (2020). A network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS-CoV-2 infection. Frontiers in Immunology, 11, 590459.
- Aloia, J. F., & Li-Ng, M. (2007). Re: Epidemic influenza and vitamin D. Epidemiology and Infection, 135(7), 1095—1096 author reply 1097—1098.
- Ames, B. N., Grant, W. B., & Willett, W. C. (2021). Does the high prevalence of vitamin D deficiency in African Americans contribute to health disparities? Nutrients, 13(2), 499.
- Ao, T., Kikuta, J., & Ishii, M. (2021). The effects of vitamin D on immune system and inflammatory diseases. Biomolecules, 11(11), 1674.
- Arboleda, J. F., Fernandez, G. J., & Urcuqui-Inchima, S. (2019). Vitamin D-mediated attenuation of miR-155 in human macrophages infected with dengue virus: Implications for the cytokine response. Infection, Genetics and Evolution, 69, 12—21.
- Ascherio, A., & Munger, K. L. (2010). Epstein-barr virus infection and multiple sclerosis: A review. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on Neurolmmune Pharmacology, 5(3), 271—277.
- Avila, E., Noriega-Mejia, B. J., Gonzalez-Macias, J., Cortes-Hernandez, U., Garcia- Quiroz, J., Garcia-Becerra, R., & Diaz, L. (2023). The preventive role of the vitamin D endocrine system in cervical cancer. International Journal of Molecular Sciences, 24(10), 8665.
- Aygun, H. (2020). Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn-Schmiedeberg's Archives of Pharmacology, 393(7), 1157—1160.
- Barreca, A. I., & Shimshack, J. P. (2012). Absolute humidity, temperature, and influenza mortality: 30 years of county-level evidence from the United States. American Journal of Epidemiology, 176(Suppl7), S114—S122.
- Bavishi, C., Maddox, T. M., & Messerli, F. H. (2020). Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiology, 5(7), 745-747.
- Belderbos, M. E., Houben, M. L., Wilbrink, B., Lentjes, E., Bloemen, E. M., Kimpen, J. L.. Bont, L. (2011). Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics, 127(6), e1513-e1520.
- Berer, A., Stockl, J., Majdic, O., Wagner, T., Kollars, M., Lechner, K., ... Oehler, L. (2000). 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Experimental Hematology, 28(5), 575-583.
- Bjornevik, K., Cortese, M., Healy, B. C., Kuhle, J., Mina, M. J., Leng, Y., ... Ascherio, A. (2022). Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science (New York, N. Y.), 375(6578), 296-301.
- Bochen, F., Balensiefer, B., Korner, S., Bittenbring, J. T., Neumann, F., Koch, A., ... Linxweiler, M. (2018). Vitamin D deficiency in head and neck cancer patients—Prevalence, prognostic value and impact on immune function. Oncoimmunology, 7(9) e1476817.
- Boehmer, T. K., Kompaniyets, L., Lavery, A. M., Hsu, J., Ko, J. Y., Yusuf, H., ... Harris, A. M. (2021). Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020-January 2021. MMWR. Morbidity and Mortality Weekly Report, 70(35), 1228-1232.
- Bont, L., Checchia, P. A., Fauroux, B., Figueras-Aloy, J., Manzoni, P., Paes, B., ... Carbonell-Estrany, X. (2016). Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infectious Diseases and Therapy, 5(3), 271-298.
- Boscoe, F. P., & Schymura, M. J. (2006). Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer, 6, 264.
- Britten, R. (1932). The incidence of epidemic influenza, 1918-19. Public Health Reports, 47(4), 303-339.
- Brown, G., Marchwicka, A., & Marcinkowska, E. (2024). Vitamin D and immune system. Vitamin D and Health. M. N. A. Eskin. Elseiver/Academic Press.
- Camargo, C. A. Jr, Ganmaa, D., Frazier, A. L., Kirchberg, F. F., Stuart, J. J., Kleinman, K., ... Rich-Edwards, J. W. (2012). Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics, 130(3), e561-e567.
- Cannell, J. J., Vieth, R., Umhau, J. C., Holick, M. F., Grant, W. B., Madronich, S., ... Giovannucci, E. (2006). Epidemic influenza and vitamin D. Epidemiology and Infection, 134(6), 1129-1140.
- Cantorna, M. T., Snyder, L., Lin, Y. D., & Yang, L. (2015). Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients, 7(4), 3011-3021.
- Castillo, J. A., & Urcuqui-Inchima, S. (2023). Vitamin D modulates inflammatory response of DENV-2-infected macrophages by inhibiting the expression of inflammatory-liked miRNAs. Pathogens and Global Health, 117(2), 167-180.
- Castillo, J. A., Giraldo, D. M., Hernandez, J. C., Smit, J. M., Rodenhuis-Zybert, I. A., & Urcuqui-Inchima, S. (2021). Regulation of innate immune responses in macrophages differentiated in the presence of vitamin D and infected with dengue virus 2. PLoS Neglected Tropical Diseases, 15(10) e0009873.
- Castillo, J. A., Giraldo, D. M., Smit, J. M., Rodenhuis-Zybert, I. A., & Urcuqui-Inchima, S. (2022). Vitamin D-induced LL-37 modulates innate immune responses of human primary macrophages during DENV-2 infection. Pathogens and Disease, 80(1) ftac014.
- Chen, G., Wu, D., Guo, W., Cao, Y., Huang, D., Wang, H., ... Ning, Q. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation, 130(5), 2620-2629.
- Chowell, G., Bertozzi, S. M., Colchero, M. A., Lopez-Gatell, H., Alpuche-Aranda, C., Hernandez, M., & Miller, M. A. (2009). Severe respiratory disease concurrent with the circulation of H1N1 influenza. The New England Journal of Medicine, 361(7), 674-679.
- Chung, C., Silwal, P., Kim, I., Modlin, R. L., & Jo, E. K. (2020). Vitamin D-cathelicidin axis: At the crossroads between protective immunity and pathological inflammation during infection. Immune Network, 20(2), e12.
- Currie, S. M., Findlay, E. G., McHugh, B. J., Mackellar, A., Man, T., Macmillan, D., ... Davidson, D. J. (2013). The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One, 8(8), e73659.
- Currie, S. M., Gwyer Findlay, E., McFarlane, A. J., Fitch, P. M., Bottcher, B., Colegrave,N., ... Davidson, D. J. (2016). Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. Journal of Immunology, 196(6), 2699-2710.
- Da Silva, E. R., Pitrez, M. C., Arruda, E., Mattiello, R., Sarria, E. E., De Paula, F. E., ... Stein, R. T. (2013). Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: Viral etiology and co-detection as risk factors. BMC Infectious Diseases, 13, 41.*Dancer, R. C., Parekh, D., Lax, S., D'Souza, V., Zheng, S., Bassford, C. R., ... Thickett, D. R. (2015). Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax, 70(7), 617-624.
- Drake, M. T., Maurer, M. J., Link, B. K., Habermann, T. M., Ansell, S. M., Micallef, I. N., ... Cerhan, J. R. (2010). Vitamin D insufficiency and prognosis in non-Hodgkin's lymphoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 28(27), 4191-4198.
- Ebrahimzadeh, A., Mohseni, S., Narimani, B., Ebrahimzadeh, A., Kazemi, S., Keshavarz, F., ... Milajerdi, A. (2021). Association between vitamin D status and risk of covid-19 inhospital mortality: A systematic review and meta-analysis of observational studies. Critical Reviews in Food Science and Nutrition, 1-11.
- Elliot, A. J., & Fleming, D. M. (2008). Influenza and respiratory syncytial virus in the elderly. Expert Review of Vaccines, 7(2), 249-258.
- Emergency Use Authorization. (2023). U.S. Food and Drug Administration.
- Entrenas Castillo, M., Entrenas Costa, L. M., Vaquero Barrios, J. M., Alcalá Díaz, J. F., López Miranda, J., Bouillon, R., & Quesada Gomez, J. M. (2020). Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. The Journal of Steroid Biochemistry and Molecular Biology, 203, 105751.
- Fang, Y., Banner, D., Kelvin, A. A., Huang, S. S., Paige, C. J., Corfe, S. A., ... Kelvin, D. J. (2012). Seasonal H1N1 influenza virus infection induces cross-protective pandemic H1N1 virus immunity through a CD8-independent, B cell-dependent mechanism. Journal of Virology, 86(4), 2229—2238.
- Ferolla, F. M., Yfran, E. W., Ballerini, M. G., Caratozzolo, A., Toledano, A., Giordano, A. C., ... Network, G. R. I. (2022). Serum vitamin D levels and life-threatening respiratory syncytial virus infection in previously healthy infants. The Journal of Infectious Diseases, 226(6), 958-966.
- Francis, M. E., McNeil, M., Dawe, N. J., Foley, M. K., King, M. L., Ross, T. M., & Kelvin, A. A. (2019). Historical H1N1 influenza virus imprinting increases vaccine protection by influencing the activity and sustained production of antibodies elicited at vaccination in ferrets. Vaccines (Basel), 7(4), 133.
- Fugl, A., & Andersen, C. L. (2019). Epstein-Barr virus and its association with disease—A review of relevance to general practice. BMC Family Practice, 20(1), 62.
- Galoppin, M., Kari, S., Soldati, S., Pal, A., Rival, M., Engelhardt, B., ... Thouvenot, E. (2022). Full spectrum of vitamin D immunomodulation in multiple sclerosis: Mechanisms and therapeutic implications. Brain Communications, 4(4) fcac171.
- Gao, N., Raduka, A., & Rezaee, F. (2023). Vitamin D(3) protects against respiratory syncytial virus-induced barrier dysfunction in airway epithelial cells via PKA signaling pathway. European Journal of Cell Biology, 102(3), 151336.
- Garvin, M. R., Alvarez, C., Miller, J. I., Prates, E. T., Walker, A. M., Amos, B. K., ... Jacobson, D. (2020). A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife, 9, e59177.
- Ghafoor, A., Siddiqi, M. R. O., Ghazanfar, A., Sarfraz, M., Shah, A. H., & Ahmad, F. (2023). Association of vitamin D with biochemical severity markers in dengue patients. PJMHS, 17(02), 88-90.
- Gibbons, J. B., Norton, E. C., McCullough, J. S., Meltzer, D. O., Lavigne, J., Fiedler, V. C., & Gibbons, R. D. (2022). Association between vitamin D supplementation and COVID-19 infection and mortality. Scientific Reports, 12(1), 19397.
- Godefroy, R., Giraud-Gatineau, A., Jimeno, M. T., Edouard, S., Meddeb, L., Zandotti, C., ... Cassir, N. (2020). Respiratory syncytial virus infection: Its propensity for bacterial coinfection and related mortality in elderly adults. Open Forum Infectious Diseases, 7(12) ofaa546.
- Gombart, A. F. (2009). The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiology, 4(9), 1151-1165.
- Gombart, A. F., Borregaard, N., & Koeffler, H. P. (2005). Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. The FASEB Journal, 19(9), 1067-1077.
- Gombash, S. E., Lee, P. W., Sawdai, E., & Lovett-Racke, A. E. (2022). Vitamin D as a risk factor for multiple sclerosis: Immunoregulatory or neuroprotective? Frontiers in Neurology, 13, 796933.
- Grant, W. B. (2008a). Hypothesis—Ultraviolet-B irradiance and vitamin D reduce the risk of viral infections and thus their sequelae, including autoimmune diseases and some cancers. Photochemistry and Photobiology, 84(2), 356-365.
- Grant, W. B. (2008b). Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. The Pediatric Infectious Disease Journal, 27(9), 853.
- Grant, W. B. (2018c). Vitamin D acceptance delayed by Big Pharma following the Disinformation Playbook. Retrieved August 4, 2022, from (http://www.orthomolecular.org/resources/ omns/v!4n22.shtml).
- Grant, W. B., & Boucher, B. J. (2022). An exploration of how solar radiation affects the seasonal variation of human mortality rates and the seasonal variation in some other common disorders. Nutrients, 14(12), 2519.
- Grant, W. B., & Garland, C. F. (2006). The association of solar ultraviolet B (UVB) with reducing risk of cancer: Multifactorial ecologic analysis of geographic variation in age- adjusted cancer mortality rates. Anticancer Research, 26(4A), 2687—2699.
- Grant, W. B., & Giovannucci, E. (2009). The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918-1919 influenza pandemic in the United States. Dermatoendocrinol, 1(4), 215—219.
- Grant, W. B., & Cannell, J. J. (2013). Autism prevalence in the United States with respect to solar UV-B doses: An ecological study. Dermatoendocrinol, 5(1), 159—164.
- Grant, W. B., Boucher, B. J., Al, F., ... Pilz (2022). Comparing the evidence from observational studies and randomized controlled trials for nonskeletal health effects of vitamin D. Nutrients, 14(18), 3811.
- Grant, W. B., Lahore, H., McDonnell, S. L., Baggerly, C. A., French, C. B., Aliano, J. L., & Bhattoa, H. P. (2020). Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients, 12(4), 988.
- Greiller, C. L., & Martineau, A. R. (2015). Modulation of the immune response to respiratory viruses by vitamin D. Nutrients, 7(6), 4240—4270.
- Gruber-Bzura, B. M. (2018). Vitamin D and influenza-prevention or therapy? International Journal of Molecular Sciences, 19(8).
- Hamid, S., Winn, A., Parikh, R., Jones, J. M., McMorrow, M., Prill, M. M., ... Hall, A. J. (2023). Seasonality of respiratory syncytial virus—United States, 2017—2023. MMWR. Morbidity and Mortality Weekly Report, 72(14), 355—361.
- Heaney, R. P. (2014). Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutrition Reviews, 72(1), 48—54.
- Hope-Simpson, R. E. (1981). The role of season in the epidemiology of influenza. Journal of Hygiene (London), 86(1), 35—47.
- Huang, C., Wang, Y., Li, X., Ren, L., Zhao,J., Hu, Y., ... Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497-506.
- Hussein, H. M., & Rahal, E. A. (2019). The role of viral infections in the development of autoimmune diseases. Critical Reviews in Microbiology, 45(4), 394-412.
- Hypponen, E., & Power, C. (2007). Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. The American Journal of Clinical Nutrition, 85(3), 860-868.
- Hypponen, E., Vimaleswaran, K. S., & Zhou, A. (2022). Genetic determinants of 25- hydroxyvitamin D concentrations and their relevance to public health. Nutrients, 14(20), 4408.
- Ianevski, A., Zusinaite, E., Shtaida, N., Kallio-Kokko, H., Valkonen, M., Kantele, A., ... Kainov, D. E. (2019). Low temperature and low UV indexes correlated with peaks of influenza virus activity in Northern Europe during 2010(-)2018. Viruses, 11(3), 207.
- Janssen, R., Bont, L., Siezen, C. L., Hodemaekers, H. M., Ermers, M. J., Doornbos, G., ... Hoebee, B. (2007). Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. The Journal of Infectious Diseases, 196(6), 826-834.
- Jartti, T., Ruuskanen, O., Mansbach, J. M., Vuorinen, T., & Camargo, C. A. Jr (2010). Low serum 25-hydroxyvitamin D levels are associated with increased risk of viral coinfections in wheezing children. The Journal of Allergy and Clinical Immunology, 126(5), 1074-1076 1076 e1071-1074.
- Jeffery, L. E., Burke, F., Mura, M., Zheng, Y., Qureshi, O. S., Hewison, M., ... Sansom, D. M. (2009). 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. Journal of Immunology, 183(9), 5458—5467.
- Jolliffe, D. A., Greenberg, L., Hooper, R. L., Mathyssen, C., Rafiq, R., de Jongh, R. T., ... Martineau, A. R. (2019). Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax, 74(4), 337-345.
- Kast, J. I., McFarlane, A. J., Globinska, A., Sokolowska, M., Wawrzyniak, P., Sanak, M., ... Wanke, K. (2017). Respiratory syncytial virus infection influences tight junction integrity. Clinical and Experimental Immunology, 190(3), 351-359.
- Kaufman, H. W., Niles,J. K., Kroll, M. H., Bi, C., & Holick, M. F. (2020). SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One, 15(9) e0239252.
- Khare, D., Godbole, N. M., Pawar, S. D., Mohan, V., Pandey, G., Gupta, S., ... Godbole, M. M. (2013). Calcitriol [1, 25[[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. European Journal of Nutrition, 52(4), 1405-1415.
- Kresfelder, T. L., Janssen, R., Bont, L., Pretorius, M., & Venter, M. (2011). Confirmation of an association between single nucleotide polymorphisms in the VDR gene with respiratory syncytial virus related disease in South African children. Journal of Medical Virology, 83(10), 1834-1840.
- Kroll, M. H., Bi, C., Garber, C. C., Kaufman, H. W., Liu, D., Caston-Balderrama, A., ... Holick, M. F. (2015). Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One, 10(3), e0118108.
- Kurtzke, J. F. (2000). Multiple sclerosis in time and space—Geographic clues to cause. Journal of Neurovirology, 6(Suppl. 2), S134-S140.
- Kusel, M. M., de Klerk, N. H., Holt, P. G., Kebadze, T.,Johnston, S. L., & Sly, P. D. (2006). Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: A birth cohort study. The Pediatric Infectious Disease Journal, 25(8), 680-686.
- Lamichhane, P. P., & Samarasinghe, A. E. (2019). The role of innate leukocytes during influenza virus infection. Journal of Immunology Research, 2019, 8028725.
- Lei, G. S., Zhang, C., Cheng, B. H., & Lee, C. H. (2017). Mechanisms of action of vitamin D as supplemental therapy for pneumocystis pneumonia. Antimicrobial Agents and Chemotherapy, 61(10) e01226-01217.
- Lemire, J. M., Adams, J. S., Kermani-Arab, V., Bakke, A. C., Sakai, R., & Jordan, S. C. (1985). 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. Journal of Immunology, 134(5), 3032-3035.
- Li-Ng, M., Aloia, J. F., PoUack, S., Cunha, B. A., Mikhail, M., Yeh,J., & Berbari, N. (2009). A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiology and Infection, 137(10), 1396-1404.
- Liu, P. T., Stenger, S., Li, H., Wenzel, L., Tan, B. H., Krutzik, S. R., ... Modlin, R. L. (2006). Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science (New York, N. Y.), 311(5768), 1770-1773.
- Loeb, M., Dang, A. D., Thiem, V. D., Thanabalan, V., Wang, B., Nguyen, N. B., ... Pullenayegum, E. (2019). Effect of Vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: A randomized controlled trial. Influenza and Other Respiratory Viruses, 13(2), 176-183.
- Lossius, A., Riise, T., Pugliatti, M., Bjornevik, K., Casetta, I., Drulovic, J., ... Holmoy, T. (2014). Season of infectious mononucleosis and risk of multiple sclerosis at different latitudes; the EnvIMS Study. Multiple Sclerosis (Houndmills, Basingstoke, England), 20(6), 669-674.
- Lowen, A. C., Mubareka, S., Steel, J., & Palese, P. (2007). Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathogens, 3(10), 1470-1476.
- Ma, J., Dushoff, J., & Earn, D. J. (2011). Age-specific mortality risk from pandemic influenza. Journal of Theoretical Biology, 288, 29—34.
- Maghbooloi, Z., Sahraian, M. A., Jaali-Moghadam, S. R., Asadi, A., Sarei, A., Zenedhdel, A., ... Holick, M. F. (2021). Treatment with 25-hydroxyvitamin D3 (calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in patients hospitalized with COVID-19: a pilot, multicenter, randomized, placebo-controlled double blind clinical trial. Endocrine Practice, 27(12) 1742—1251.
- Mansbach, J. M., Hasegawa, K., Ajami, N. J., Petrosino, J. F., Piedra, P. A., Tierney, C. N., ... Camargo, C. A. (2017). Serum LL-37 levels associated with severity of bronchiolitis and viral etiology. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 65(6), 967—975.
- Marik, P. E., Kory, P., Varon,J., Iglesias, J., &Meduri, G. U. (2021). MATH+ protocolfor the treatment of SARS-CoV-2 infection: The scientific rationale. Expert Review of Anti- Infective Therapy, 19(2), 129—135.
- Marr, L. C., Tang, J. W., Van, J., ... Lakdawala (2019). Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. Journal of the Royal Society, Interface/the Royal Society, 16(150), 20180298.
- Martina, B. E., Koraka, P., & Osterhaus, A. D. (2009). Dengue virus pathogenesis: An integrated view. Clinical Microbiology Reviews, 22(4), 564—581.
- Martineau, A. R., Jolliffe, D. A., Hooper, R. L., Greenberg, L., Aloia, J. F., Bergman, P., ... Camargo, C. A. Jr. (2017). Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ (Clinical Research ed.), 356, i6583.
- Martinez-Moreno, J., Hernandez, J. C., & Urcuqui-Inchima, S. (2020). Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Molecular and Cellular Biochemistry, 464(1-2), 169-180.
- McNally, J. D., Sampson, M., Matheson, L. A., Hutton, B., & Little, J. (2014). Vitamin D receptor (VDR) polymorphisms and severe RSV bronchiolitis: A systematic review and meta-analysis. Pediatric Pulmonology, 49(8), 790-799.
- Meftahi, G. H., Jangravi, Z., Sahraei, H., & Bahari, Z. (2020). The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: The contribution of "inflame-aging. Inflammation Research: Official Journal of the European Histamine Research Society. [et al.], 69(9), 825-839.
- Mercola, J., Grant, W. B., & Wagner, C. L. (2020). Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients, 12(11), 3361.
- Moore, P. S., & Chang, Y. (2010). Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nature Reviews. Cancer, 10(12), 878-889.
- Munoz, A., & Grant, W. B. (2022). Vitamin D and cancer: An historical overview of the epidemiology and mechanisms. Nutrients, 14(7), 1448.
- Obando-Pacheco, P., Justicia-Grande, A. J., Rivero-Calle, I., Rodriguez-Tenreiro, C., Sly, P., Ramilo, O., ... Martinon-Torres, F. (2018). Respiratory syncytial virus seasonality: A global overview. The Journal of Infectious Diseases, 217(9), 1356-1364.
- Ohaegbulam, K. C., Swalih, M., Patel, P., Smith, M. A., & Perrin, R. (2020). Vitamin D supplementation in COVID-19 patients: A clinical case series. American Journal of Therapeutics, 27(5), e485-e490.
- Olsen, S. J., Azziz-Baumgartner, E., Budd, A. P., Brammer, L., Sullivan, S., Pineda, R. F., Cohen, C., & Fry, A. M. (2020). Decreased influenza activity during the COVID-19 pandemic-United States, Australia, Chile, and South Africa, 2020. American Journal of Transplantation: Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 20(12), 3681-3685.
- Oristrell, J., Oliva, J. C., Casado, E., Subirana, I., Dominguez, D., Toloba, A., Balado, A., & Grau, M. (2022). Vitamin D supplementation and COVID-19 risk: A population- based, cohort study. Journal of Endocrinological Investigation, 45(1), 167—179.
- Ostkamp, P., Salmen, A., Pignolet, B., Gorlich, D., Andlauer, T. F. M., Schulte- Mecklenbeck, A., ... German, S. Competence Network Multiple and B. N. the. (2021). Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proceedings of the National Academy of Sciences of the United States of America, 118(1) e2018457118.
- Pavia, A. T. (2011). Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 52 (Suppl. 4), S284—S289.
- Penna, G., & Adorini, L. (2000). 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. Journal of Immunology, 164(5), 2405—2411.
- Puerta-Guardo, H., Medina, F., De la Cruz Hernandez, S. I., Rosales, V. H., Ludert, J. E., & del Angel, R. M. (2012). The 1alpha,25-dihydroxy-vitamin D3 reduces dengue virus infection in human myelomonocyte (U937) and hepatic (Huh-7) cell lines and cytokine production in the infected monocytes. Antiviral Research, 94(1), 57—61.
- Rhodes, J. M., Subramanian, S., Laird, E., Griffin, G., & Kenny, R. A. (2021). Perspective: Vitamin D deficiency and COVID-19 severity—Plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. Journal of Internal Medicine, 289(1), 97—115.
- Rolf, J. D. (2020). Clinical characteristics of Covid-19 in China. The New England Journal of Medicine, 382(19), 1860.
- Rose, E. B., Wheatley, A., Langley, G., Gerber, S., & Haynes, A. (2018). Respiratory syncytial virus seasonality—United States, 2014—2017. MMWR. Morbidity and Mortality Weekly Report, 67(2), 71—76.
- Ross, A. C., Manson, J. E., Abrams, S. A., Aloia, J. F., Brannon, P. M., Clinton, S. K., Durazo-Arvizu, R. A., Gallagher, J. C., Gallo, R. L., Jones, G., Kovacs, C. S., Mayne, S. T., Rosen, C. J., & Shapses, S. A. (2011). The 2011 dietary reference intakes for calcium and vitamin D: What dietetics practitioners need to know. Journal of the American Dietetic Association, 111(4), 524—527.
- Rossi, G. A., Fanous, H., & Colin, A. A. (2020). Viral strategies predisposing to respiratory bacterial superinfections. Pediatric Pulmonology, 55(4), 1061—1073.
- Schwalfenberg, G. K. (2011). A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Molecular Nutrition & Food Research, 55(1), 96—108.
- Shahbaz, U., Fatima, N., Basharat, S., Bibi, A., Yu, X., Hussain, M. I., & Nasrullah, M. (2022). Role of vitamin C in preventing of COVID-19 infection, progression and severity. AIMS Microbiology, 8(1), 108—124.
- Shaman, J., & Kohn, M. (2009). Absolute humidity modulates influenza survival, transmission, and seasonality. Proceedings of the National Academy of Sciences of the United States of America, 106(9), 3243-3248.
- Sharifi, A., Vahedi, H., Nedjat, S., Rafiei, H., & Hosseinzadeh-Attar, M. J. (2019). Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: A randomized placebo-controlled trial. APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica, 127(10), 681-687.
- Shim, J., Perez, A., Symanski, E., & Nyitray, A. G. (2016). Association between serum 25- hydroxyvitamin D level and human papillomavirus cervicovaginal infection in women in the United States. The Journal of Infectious Diseases, 213(12), 1886-1892.
- Sirbu, A. C., Sabin, O., Bocsan, I. C., Vesa, S. C., & Buzoianu, A. D. (2023). The effect of vitamin D supplementation on the length ofhospitalisation, intensive care unit admission, and mortality in COVID-19—A systematic review and meta-analysis. Nutrients, 15(15), 3470.
- Smolders, J., van den Ouweland, J., Geven, C., Pickkers, P., & Kox, M. (2021). Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism, 115, 154434.
- Smolders, J., Torkildsen, O., Camu, W., & Holmoy, T. (2019). An update on vitamin D and disease activity in multiple sclerosis. CNS Drugs, 33(12), 1187—1199.
- Starska-Kowarska, K. (2023). Role of vitamin D in head and neck cancer-immune function, anti-tumour effect, and its impact on patient prognosis. Nutrients, 15(11).
- Su, T. H., Liu, C.J., Tseng, T. C., Chou, S. W., Liu, C. H., Yang, H. C., Wu, S.J., Chen, P. J., Chen, D. S., Chen, C. L., & Kao, J. H. (2019). Chronic hepatitis B is associated with an increased risk of B-cell non-Hodgkin's lymphoma and multiple myeloma. Alimentary Pharmacology & Therapeutics, 49(5), 589—598.
- Sundaresan, B., Shirafkan, F., Ripperger, K., & Rattay, K. (2023). The role of viral infections in the onset of autoimmune diseases. Viruses, 15(3).
- Thandra, K. C., Barsouk, A., Saginala, K., Padala, S. A., Barsouk, A., & Rawla, P. (2021). Epidemiology of Non-Hodgkin's Lymphoma. Medical Sciences (Basel), 9(1), 5.
- Tin Tin Htar, M., Yerramalla, M. S., Moisi, J. C., & Swerdlow, D. L. (2020). The burden of respiratory syncytial virus in adults: A systematic review and meta-analysis. Epidemiology and Infection, 148, e48.
- Tripkovic, L., Wilson, L. R., Hart, K., Johnsen, S., de Lusignan, S., Smith, C. P., Bucca, G., Penson, S., Chope, G., Elliott, R., Hypponen, E., Berry, J. L., & Lanham-New, S. (2017). Daily supplementation with 15 mug vitamin D2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: A 12-wk randomized, placebo-controlled food-fortification trial. The American Journal of Clinical Nutrition, 106(2), 481—490.
- Urashima, M., Mezawa, H., Noya, M., & Camargo, C. A. Jr. (2014). Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food & Function, 5(9), 2365—2370.
- Urashima, M., Segawa, T., Okazaki, M., Kurihara, M., Wada, Y., & Ida, H. (2010). Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. The American Journal of Clinical Nutrition, 91(5), 1255—1260.
- Vaghari-Tabari, M., Mohammadzadeh, I., Qujeq, D., Majidinia, M., Alemi, F., Younesi, S., Mahmoodpoor, A., Maleki, M., Yousefi, B., & Asemi, Z. (2023). Vitamin D in respiratory viral infections: A key immune modulator? Critical Reviews in Food Science and Nutrition, 63(14), 2231—2246.
- Vandebergh, M., Dubois, B., & Goris, A. (2022). Effects of vitamin D and body mass index on disease risk and relapse hazard in multiple sclerosis: A mendelian randomization study. Neurology Neuroimmunology & Neuroinflammation, 9(3), e1165.
- Vanegas-Cedillo, P. E., Bello-Chavolla, O. Y., Ramirez-Pedraza, N., Rodriguez Encinas, , Perez Carrion, C. I., Jasso-Avila, M. I., ... Mehta, R. (2022). Serum vitamin D levels are associated with increased COVID-19 severity and mortality independent of whole- body and visceral adiposity. Frontiers in Nutrition, 9, 813485.
- Vassiliou, A. G., Jahaj, E., Pratikaki, M., Keskinidou, C., Detsika, M., Grigoriou, E., Psarra, K., Orfanos, S. E., Tsirogianni, A., Dimopoulou, I., & Kotanidou, A. (2021). Vitamin D deficiency correlates with a reduced number of natural killer cells in intensive care unit (ICU) and non-ICU patients with COVID-19 pneumonia. Hellenic Journal of Cardiology: HJC = Hellenike Kardiologike Epitheorese, 62(5), 381—383.
- Villamor, E., Villar, L. A., Lozano, A., Herrera, V. M., & Herran, O. F. (2017). Vitamin D serostatus and dengue fever progression to dengue hemorrhagic fever/dengue shock syndrome. Epidemiology and Infection, 145(14), 2961—2970.
- Visser, E., Milne, D., Collacott, I., McLernon, D., Counsell, C., & Vickers, M. (2014). The epidemiology ofinfectious mononucleosis in Northern Scotland: A decreasing incidence and winter peak. BMC Infectious Diseases, 14, 151.
- Vitkova, M., Diouf, I., Malpas, C., Horakova, D., Kubala Havrdova, E., Patti, F., ... Group, M. S. S. (2022). Association of latitude and exposure to ultraviolet B radiation with severity of multiple sclerosis: An international registry study. Neurology, 98, e2401—e2412.
- Vo, P., Koppel, C., Espinola, J. A., Mansbach, J. M., Celedon, J. C., Hasegawa, K., Bair- Merritt, M., & Camargo, C. A. Jr (2018). Vitamin D status at the time of hospitalization for bronchiolitis and its association with disease severity. The Journal of Pediatrics, 203(416-422), e411.
- Vos, L. M., Bruyndonckx, R., Zuithoff, N. P. A., Little, P., Oosterheert, J. J., Broekhuizen, B. D. L., ... Consortium, G. (2021). Lower respiratory tract infection in the community: Associations between viral aetiology and illness course. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases, 27(1), 96-104.
- Wang, T. T., Nestel, F. P., Bourdeau, V., Nagai, Y., Wang, Q., Liao, J., Tavera-Mendoza, L., Lin, R., Hanrahan, J. W., Mader, S., & White, J. H. (2004). Cutting edge: 1,25- dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of Immunology, 173(5), 2909-2912.
- Watad, A., Azrielant, S., Bragazzi, N. L., Sharif, K., David, P., Katz, I., Aljadeff, G., Quaresma, M., Tanay, G., Adawi, M., Amital, H., & Shoenfeld, Y. (2017). Seasonality and autoimmune diseases: The contribution of the four seasons to the mosaic of autoimmunity. Journal of Autoimmunity, 82, 13-30.
- Webb, A. R., Kline, L., & Holick, M. F. (1988). Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. The Journal of Clinical Endocrinology and Metabolism, 67(2), 373-378.
- Weller, R. B., Mahrhofer, M., Davis, W., & Gorman, S. (2021). Risks and benefits of UV radiation. Current Problems in Dermatology, 55, 329-338.
- White, J. H. (2012). Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Archives of Biochemistry and Biophysics, 523(1), 58-63.
- Williams, P. C. M., Howard-Jones, A. R., Hsu, P., Palasanthiran, P., Gray, P. E., McMullan, B. J., Britton, P. N., & Bartlett, A. W. (2020). SARS-CoV-2 in children: Spectrum of disease, transmission and immunopathological underpinnings. Pathology, 52(7), 801-808.
- Wimalawansa, S. J. (2022). Rapidly increasing serum 25(OH)D boosts the immune system, against infections-sepsis and COVID-19. Nutrients, 14(14), 2997.
- Wimalawansa, S. J. (2023). Physiological basis for using vitamin D to improve health. Biomedicines, 11 (6).
- Woese, C. (1960). Thermal inactivation of animal viruses. Annals of the New York Academy of Sciences, 83, 741-751.
- Xu, J., Yang, J., Chen, J., Luo, Q., Zhang, Q., & Zhang, H. (2017). Vitamin D alleviates lipopolysaccharideinduced acute lung injury via regulation of the reninangiotensin system. Molecular Medicine Reports, 16(5), 7432-7438.
- Yan, T., Xiao, R., & Lin, G. (2020). Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: A double-edged sword? The FASEBJournal, 34(5), 6017-6026.
- Yanez, N. D., Weiss, N. S., Romand, J. A., & Treggiari, M. M. (2020). COVID-19 mortality risk for older men and women. BMC Public Health, 20(1), 1742.
- Yang, X., Quam, M. B. M., Zhang, T., & Sang, S. (2021). Global burden for dengue and the evolving pattern in the past 30 years. Journal of Travel Medicine: Official Publication of the International Society of Travel Medicine and the Asia Pacific Travel Health Association, 28(8) taab146.
- Yorita, K. L., Holman, R. C., Steiner, C. A., Efiler, P. V., Miyamura, J., Forbes, S., Anderson, L. J., & Balaraman, V. (2007). Severe bronchiolitis and respiratory syncytial virus among young children in Hawaii. The Pediatric Infectious Disease Journal, 26(12), 1081-1088.
- Yuan, S., Xiong, Y., & Larsson, S. C. (2021). An atlas on risk factors for multiple sclerosis: A Mendelian randomization study. Journal of Neurology, 268(1), 114—124.
- Yusuf, S., Piedimonte, G., Auais, A., Demmler, G., Krishnan, S., Van Caeseele, P., ... Welliver, R. (2007). The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiology and Infection, 135(7), 1077—1090.
- Zhou, J., Du, J., Huang, L., Wang, Y., Shi, Y., & Lin, H. (2018). Preventive effects of vitamin D on seasonal influenza A in infants: A multicenter, randomized, open, controlled clinical trial. The Pediatric Infectious Disease Journal, 37(8), 749—754.
- Zhu, Z., Zhu, X., Gu, L., Zhan, Y., Chen, L., & Li, X. (2021). Association between vitamin D and influenza: Meta-analysis and systematic review of randomized controlled trials. Frontiers in Nutrition, 8, 799709.
- Zur Hausen, H. (1991). Viruses in human cancers. Science (New York, N. Y.), 254(5035), 1167-1173.
55+ papers By Dr. Grant in VitaminDWiki
VitaminDWiki – COVID-19 treated by Vitamin D - studies, reports, videos
As of March 31, 2024, the VitaminDWiki COVID page had: trial results, meta-analyses and reviews, Mortality studies see related: Governments, HealthProblems, Hospitals, Dark Skins, All 26 COVID risk factors are associated with low Vit D, Fight COVID-19 with 50K Vit D weekly Vaccines Take lots of Vitamin D at first signs of COVID 166 COVID Clinical Trials using Vitamin D (Aug 2023) Prevent a COVID death: 9 dollars of Vitamin D or 900,000 dollars of vaccine - Aug 2023
5 most-recently changed Virus entries
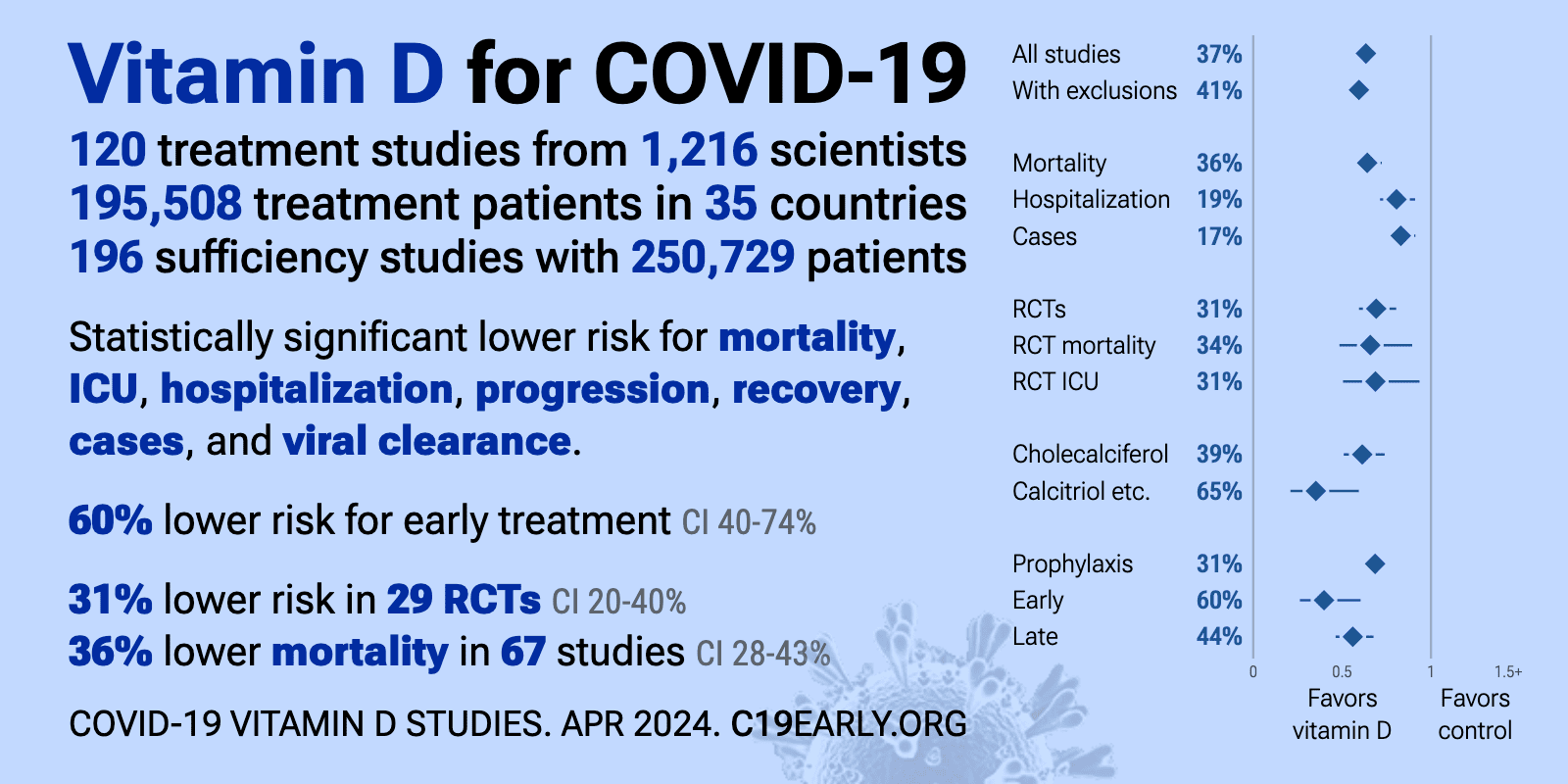
- The above image is automatically updated
VitaminDWiki – Overview Influenza and vitamin D
VitaminDWiki – Respiratory viral infection (RSV) and low vitamin D - many studies
VitaminDWiki – Infections and low vitamin D - many studies
VitaminDWiki – Dengue Fever decimated by Vitamin D - many studies
VitaminDWiki – Epstein-Barr Virus probably causes Long-COVID, CFS, and MS - many studies
VitaminDWiki – Cancer category contains:
- Cancer
295 items Overview Cancer and vitamin D - Cancer and Vitamin D - many studies
- After Cancer Diagnosis
113 items - Bladder Cancer
28 items - Breast Cancer
259 items Overview Breast Cancer and Vitamin D - Colon Cancer
145 items Overview Cancer-Colon and vitamin D - Leukemia
19 items - Liver Cancer
17 items - Lung Cancer
55 items Overview Lung cancer and vitamin D - Lymphoma Cancer
26 items - Other Cancer
66 items - Ovarian Cancer
26 items - Pancreatic Cancer
58 items - Prostate Cancer
103 items Overview Prostate Cancer and Vitamin D - Skin Cancer
121 items Overview Suntan, melanoma and vitamin D - Childhood Cancers - Vitamin D can help - many studies
- Easiest way to treat cancer – take Vitamin D – Nov 2022
- 13 Cancers are helped by Vitamin D – Biobank July 2023
- 2X increase of 14 cancers in non-seniors in 20 years (low vitamin D) – Sept 2022
- Vitamin D prevents and treats cancer in many ways – May 2021
- Those with recent cancer diagnosis had 7X increased risk of COVID-19 (more if A-A )- Dec 2020
- Deaths from many types of Cancer associated with low vitamin D- review of meta-analyses Sept 2020
- Cancer incidence and mortality is decreased if 40-60 ng of Vitamin D – April 2019
- 8 ways that Cancer might be prevented by Vitamin D - June 2019
- Cancer stem cells and Vitamin D - many studies
- Vitamin D Reduces Cancer Risk - Why Scientists Accept It but Physicians Do Not - Feb 2019
- Overview of Vitamin D Actions in Cancer – 31 page chapter in a book – 2018
- Vitamin D prevents breast cancer, reduces BC mortality, and reduces BC chemotherapy problems – Sept 2018
- Diagnosed with breast cancer – take vitamin D to cut chance of death by half – July 2018
- Melanoma 25 X more likely if low vitamin D – Feb 2018
- Better Cancer survival if higher vitamin D a decade earlier (esp. Melanoma, Kidney, Prostate)– Aug 2018
Cancers get less Vitamin D when there is a poor Vitamin D Receptor- Vitamin D Receptor pages in VitaminDWiki with CANCER in title 86 as of July 2023
- Cancer and the Vitamin D Receptor, a primer – Sept 2017
- Vitamin D Receptor (Cancers OR Viruses) - many studies
- Risk of Cancer increased if poor Vitamin D Receptor – meta-analysis of 73 studies Jan 2016
- Cancer (general) and VDR
23 articles - Breast Cancer and VDR
24 articles - Colon Cancer and VDR
13 articles - Prostate Cancer and VDR
7 articles - Skin Cancer and VDR
10 articles - Note some Health problems, such as some Cancers, protect themselves
by actively reducing Receptor activation
11+ VitaminDWiki Virus pages have CANCER in the title
This list is automatically updated
Vitamin D: Viral infections, Infectious diseases, EBV and MS, Virus and Cancers – Grant March 2024(Cached) Printer Friendly Follow this page for updates7610 visitors, last modified 03 Mar, 2024, This page is in the following categories (# of items in each category) - Breast Cancer and VDR