Rheumatoid arthritis and many other diseases associated with low Boron – 2012
Serum boron concentration in rheumatoid arthritis: correlation with disease activity, functional class, and rheumatoid factor
Journal of Experimental and Integrative Medicine Online Dec 22, 2012, DOI 10.5455/jeim.101112.or.053
Ziad S. Al-Rawi1, Faiq I. Gorial1, Wejdi A. Al-Shammary2, Fadhil Muhsin3, Ahmed S. Al-Naaimi4, Sa’ad Kareem3
1 Department of Medicine, and 4Department of Community Medicine; College of Medicine; University of Baghdad; 2Baghdad Teaching Hospital, Rheumatology Unit; and 3Iraqi Ministry of Science and Technology; Baghdad, Ira
Corresponding Author: Faiq I. Gorial, Department of Medicine, College of Medicine, University of Baghdad, Baghdad, Iraq. faiqig@yahoo.com
📄 Download the PDF from VitaminDWiki
Objectives: Rheumatoid arthritis (RA) is a common chronic inflammatory arthropathy of unknown etiology. Trace elements have a great role in a number of biological processes. The aim of this study was to assess the serum element boron in a sample of Iraqi patients with RA and to evaluate its relationship if present with disease activity, functional class of the disease, and rheumatoid factor (RF).
Methods: A cross sectional study enrolled 107 RA patients and 214 controls matched in age and sex. The American College of Rheumatology 1987 revised criteria was used for diagnosis of RA. Disease Activity Score index of 28 joints (DAS28), functional class of RA patients, RF, erythrocyte sedimentation rate (ESR) were measured in patients’ group; serum boron levels were measured using a flame atomic absorption spectrophotometer in both patients and controls groups.
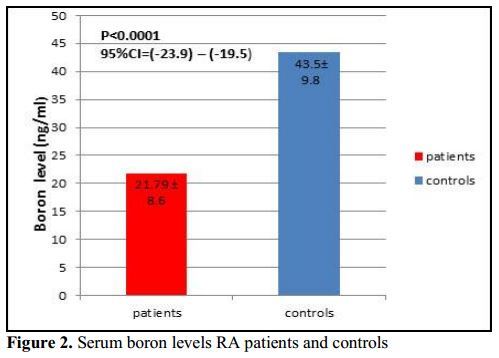
Results: RA patients had significantly lower serum boron level than controls (P < 0.001). Serum boron level was significantly negatively correlated with RF titer in RA patients (r = -0.22, P = 0.001). No significant correlation was found between serum boron with DAS28, disease functional class, and ESR (P > 0.05). Also, RF titer was a significant predictor of low serum boron level (P = 0.023 , OR = -0.07, 95%CI -0.13-(-0.01)).
Conclusions: There was a significant low serum boron level in RA patients. RF titer was significant predictor of low serum boron level. This may suggest that boron element may play a role in pathophysiology of RA and its severity. Supplementation with boron element and diets rich in fruits, vegetables, nuts, and pulses may be useful.
DISCUSSION (from PDF)
The importance of trace elements in chronic inflammatory diseases is related to their cofactor role in immune system functions and in different metabolic processes in articular tissues [17].
A variety of trace elements are found in bones including iron (Fe), copper (Cu), zinc (Zn), manganese (Mn), fluoride (F), strontium (Sr), and boron (B) [18]. Although they are present in only minute amounts, trace elements influence normal metabolic processes through interaction with -or incorporation into- proteins, particularly enzymes [19].
Growing evidence from a variety of experimental models shows that boron is a bioactive and beneficial element for humans.
Reported beneficial actions of boron include
arthritis alleviation or risk reduction,
bone growth and maintenance,
central nervous system function,
cancer risk reduction,
hormone facilitation, and immune response,
inflammation, and oxidative stress modulation.
Formation of boroesters with the ribose moiety of compounds involved in numerous reactions, such as S-adenosylmethionine and oxidized nicotinamide adenine dinucleotide (NAD+) might be the reason for boron bioactivity [20].
The role of boron is particularly important because it controls the inflammatory process in arthritic conditions by down-regulating specific enzymatic activities typically elevated during inflammation at the inflammatory site [21], inhibiting the inflammatory stress [22], and affecting the production of inflammatory cytokines by cartilage cells and cells involved in the inflammatory response [23].
This study showed that serum boron level was significantly lower in RA patients (21.79 ± 8.6) compared to controls (43.5 ± 9.8). Havercroft and Ward [24] reported that boron concentrations in bone and synovial fluid were lower in RA patients than in healthy controls.
Newnham [25] reported that the occurrence of arthritis is negatively correlated with the amount of boron in the soil and in the food and water supply. In areas where daily boron intakes were typically 1 mg, the estimated incidence of arthritis ranged from 20% to 70%. In areas where daily boron intakes ranged from 3 to >10 mg, the estimated incidence of arthritis ranged from 0% to 10%.
A recent study of 20 patients with mild, moderate, or severe osteoarthritis also found that boron supplementation alleviated subjective measures of arthritis [26]. Patients with mild to moderate arthritis supplemented daily with 6 mg of boron as calcium fructoborate (a naturally occurring boron complex commonly found in fruits and vegetables) reported markedly reduced pain. By week 8, 80% of the test participants reduced or eliminated their use of painkillers. In addition, joint rigidity essentially disappeared, and mobility was markedly increased at 8 weeks. Patients with severe arthritis, who were supplemented daily with 12 mg of boron as calcium fructoborate, exhibited a more subdued improvement in mobility and rigidity but still reported a significant reduction in the use of painkillers. These findings, however, are weakened by the non-blinding to treatment and lack of placebo controls.
Additionally, another study in juvenile idiopathic arthritis (JIA) reported that boron supplementation at 3-9 mg per day may be beneficial [27]. A finding of note in this study was that serum boron level was not significantly correlated with disease activity, functional class, and ESR of RA patients; this may be explained by medications used in RA that control disease activity and subsequently improves activity and functional class.
Interestingly, the current study showed significant negative correlation between serum boron level and RF, a predictive of more aggressive and erosive articular disease and poorer long-term function in RA patients [28]; this may suggest that boron deficiency can be associated with the severity of RA disease.
The small size of the studied sample and short period of the study were the main limitations of the present study and these can be increased in larger scales, multicenter prospective studies, with longer period of follow up to support the reported data. Yet, in spite of that, this study has points of strength like strict inclusion and exclusion criteria, and defined data measurement and collection.
In conclusion, serum boron level was significantly lower in RA patients compared to controls and significantly negatively correlated with RF in RA patients. This may indicate that B element may play a role in pathophysiology of RA and its severity which is clinically relevant and suggest the importance of boron element supplementation to RA patients and even more important to individuals who are at high risk of developing RA.
REFERENCES
Fan LY, Zong M, Wang Q, Yang L, Sun LS, Ye Q, Ding YY, Ma JW.. Diagnostic value of glucose-6-phosphate isomerase in rheumatoid arthritis. Clin Chim Acta 2010; 411:2049-53.
Jaswal S, Mehta HC, Sood AK, Kaur J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta 2003 ; 338:123-9.
Staib A, Dolenko B, Fink DJ, Fruh J, Nikulin AE, Otto M, Pessin-Minsley MS, Quarder O, Somorjai R, Thienel U, Werner G, Petrich W.. Disease pattern recognition testing for rheumatoid arthritis using infrared spectra of human serum. Clin Chim Acta 2001; 308:79-89.
Nijenhuis S, Zendman AJ, Vossenaar ER, Pruijn GJ, vanVenrooij WJ. Autoantibodies to citrullinated proteins in rheumatoid arthritis: clinical performance and biochemical aspects of an RA-specific marker. Clin Chim Acta 2004; 350:17 34.
King DE, Mainous AG, Geesey ME, Woolson RF. Dietary magnesiumand C-reactive protein levels. J Am Coll Nutr 2005; 24:166-71
Zampieron ER, Kamhi E. Arthritis: An Alternative Medicine Definitive Guide, 2nd edition, Celestial Arts, Berkeley, CA, USA, 2006.
Lovell DJ, Glass D, Ranz J, Kramer S, Huang B, Sierra RI, Henderson CJ, Passo M, Graham B, Bowyer S, Higgins G, Rennebohm R, Schikler KN, Giannini E.. A randomized controlled trial of calcium supplementation to increase bone mineral density in children with juvenile rheumatoid arthritis. Arthrit Rheum 2006; 54:2235-42.
Pasha Q, Malik SA, Shaheen N, Shah MH. Investigation of trace metals in the blood plasma and scalp hair of gastrointestinal cancer patients in comparison with controls. Clin Chim Acta 2010; 411:531-9.
Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition 2004; 20:632-44.
Hunt CD. Dietary boron : an overview of the evidence for its role in immune function. J Trace Elem Exp Med 2003, 16:291-306.
Devirian TA, Volpe SL. The physiological effects of dietary boron . Crit Rev Food Sci Nutr 2003; 43:219-31.
Taneja SK, Mandal R. Assessment of mineral status (Zn, Cu, Mg and Mn) in rheumatoid arthritis patients in Chandigarh, India. Rheumatology Reports 2009; 1(e5):16-20.
Colak M, Bingol NK, Ayhan O, Avci S, Bulut V. Serum copper, zinc and selenium levels in rheumatoid arthritis. Romatizma 2001; 16:66-71.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315-24.
Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modi. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38:44-8.
Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992; 35:498-502.
Ala S, Shokrzadeh M, Pur Shoja AM, Saeedi Saravi SS. Zinc and copper plasma concentrations in rheumatoid arthritis patients from a selected population in Iran. Pak J Biol Sci 2009; 12:1041 4.
Sandstead HH, Penland JG, Alcock NW, Dayal HH, Chen XC, Li JS, Zhao F, Yang JJ. Effects of repletion with zinc and other micronutrients on neuropsychologic performance and growth of Chinese children. Am J Clin Nutr 1998; 68:470-5S.
Grynpas MD. Fluoride effects on bone crystals. J Bone Mineral Res 1990; 5:S169-75.
Nielsen FH, Meacham SL. Growing evidence for human health benefits of boron . J Evid Based Complement Alternat Med 2011 16:169-80.
Sutherland B, Strong P, King JC. Determining human dietary requirements for boron . Biol Trace Elem Res 1998; 66:193-204.
Hunt CD, Idso JP. Dietary boron as a physiological regulator of the normal inflammatory response: a review and current research progress. J Trace Elem Exp Med. 1999; 12:221-33.
Benderdour M, Hess K, Dzondo-Gadet M, Nabet P, Belleville F, Dousset B. B modulates extracellular matrix and TNFa synthesis in human fibroblasts. Biochem Biophys Res Commun 1998; 246:746-51.
Havercroft JM, Ward NI. B and other elements in relation to rheumatoid arthritis. In: Momcilovic B (ed) Trace Elements in Man and Animals 7. IMI, Zagreb, Croatia, pp 8.2-8.3, 1991.
Newnham RE. How boron is being used in medical practice. In: Goldbach HE, Rerkasem B, Wimmer MA, Brown PH, Thellier M, Bell RW (eds) B in Plant and Animal Nutrition. Kluwer, NewYork, NY, USA, pp 59-62, 2002.
Miljkovic D, Scorei RI, Cimpoiasu VM, Scorei ID. Calcium fructoborate: plant-based dietary boron for human nutrition. J Diet Suppl 2009; 6:211-26.
Newnham RE. Arthritis or skeletal fluorosis and boron . Int Clin Nutr Rev 1991; 11:68-70.
Tehlirian CV, Bathon JM. Rheumatoid arthritis. In: Klippel JH, Stone JH, Crofford LJ, White PH (eds) Primer on the Rheumatic Diseases, 13th edition, Springer, New York, NY, USA, pp 118119, 2008.
