FTC does not allow any advertising claims of Vitamin D for COVID (but more than 100 studies have found it helps)
Nutrional Outlook July 22, 2021
“Express claims including the
words SARS-CoV-02 or COVID-19 are not allowed.
Neither are claims in which effects on COVID-19 are implied,
- including claims of “supports immune function”
followed by the hashtag #COVID-19 or
even an image of the virus,
citations of studies assessing the virus,
or the word pandemic.
References to signs and symptoms of COVID-19 also aren’t allowed,
such as “alleviates shortness of breath and inflammation,”
or claims about the body’s response to the vector, such as “reduces viral replication.”
Finally, a supplement product name should not imply a connection to the disease, such as “CovRx” or “CovAway,” she said.”
“Cleland also called out vitamin D specifically . “With regard to vitamin D, the supporting evidence is mostly observational, and the scanty clinical evidence is inconsistent and flawed,” he stated. “Moreover, the relevant scientific question is not necessarily whether there is an association between vitamin D and the severity of COVID symptoms, but assuming that there is an association, whether supplementation has any effect and, if so, when does it have to be administered to be effective and what dose is effective? These are all questions of which currently there are no answers for.”
Far more studies of the efficacy and safety of Vitamin D than for vaccines
Vitamin D has been found to be safe for use in 3rd generation,
vs 1 year of limited safety information on COVID-19 vaccines
COVID-19 treated by Vitamin D - studies, reports, videos
{include}
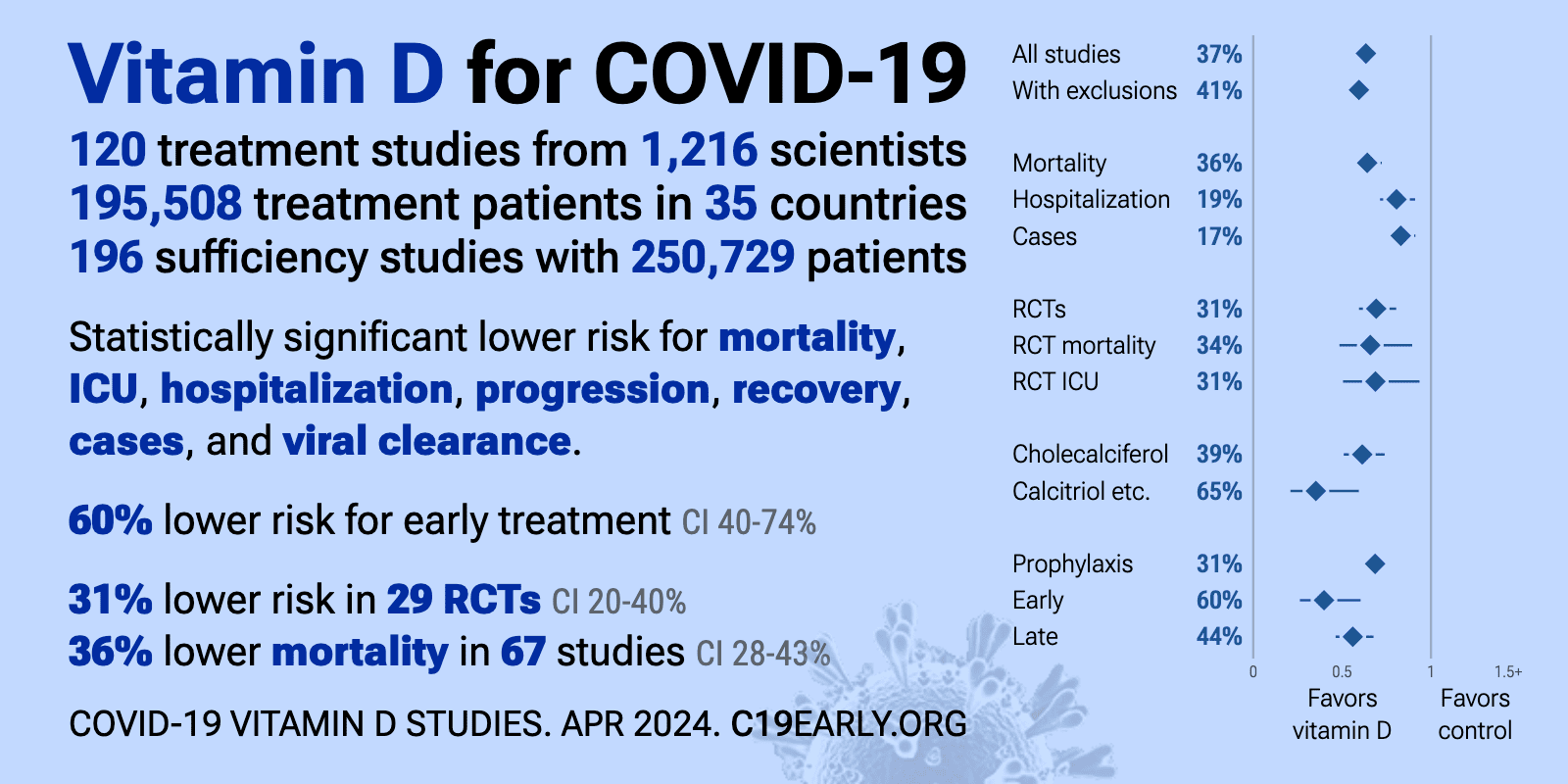
- The above image is automatically updated
26 health factors increase the risk of COVID-19 – all are proxies for low vitamin D
Mortality and Virus studies
{category}
Vitamin D meta-analyses for Virus
{category}
