There may be a bias against Vitamin C in Mainstream Medicine
Bias against Vitamin C in Mainstream Medicine: Examples from Trials of Vitamin C for Infections
Life 2022, 12(1), 62; https://doi.org/10.3390/life12010062
by Harri Hemilä 1,*ORCID and Elizabeth Chalker 2
1 Department of Public Health, University of Helsinki, FI-00014 Helsinki, Finland
2 Biological Data Science Institute, Australian National University, Canberra, ACT 2600, Australia
PDF Table of Contents
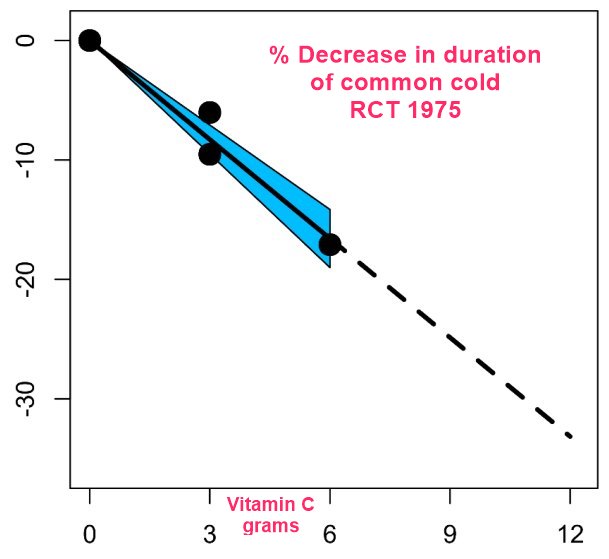
Reduced duration of common cold with increasing Vitamin C
Evidence has shown unambiguously that, in certain contexts, vitamin C is effective against the common cold. However, in mainstream medicine, the views on vitamin C and infections have been determined by eminence-based medicine rather than evidence-based medicine. The rejection of the demonstrated benefits of vitamin C is largely explained by three papers published in 1975—two published in JAMA and one in the American Journal of Medicine—all of which have been standard citations in textbooks of medicine and nutrition and in nutritional recommendations. Two of the papers were authored by Thomas Chalmers, an influential expert in clinical trials, and the third was authored by Paul Meier, a famous medical statistician. In this paper, we summarize several flaws in the three papers. In addition, we describe problems with two recent randomized trial reports published in JAMA which were presented in a way that misled readers. We also discuss shortcomings in three recent JAMA editorials on vitamin C. While most of our examples are from JAMA, it is not the only journal with apparent bias against vitamin C, but it illustrates the general views in mainstream medicine. We also consider potential explanations for the widespread bias against vitamin C.
📄 Download the PDF from VitaminDWiki
References
Pauling, L. The significance of the evidence about ascorbic acid and the common cold. Proc. Natl. Acad. Sci. USA 1971, 68,2678-2681. [CrossRef]
Pauling, L. Ascorbic acid and the common cold. Am. J. Clin. Nutr. 1971, 24,1294-1299. [CrossRef] [PubMed]
Pauling, L. Vitamin C and the Common Cold; Freeman: San Francisco, CA, USA, 1970.
Hemila, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 2013, CD000980. [CrossRef]
Hemila, H.; Chalker, E. Vitamin C for Preventing and Treating the Common Cold. Available online: http://www.mv.helsinki.fi/ home/hemila/CC (accessed on 14 December 2021).
Shamseer, L.; Vohra, S.; Bax, R.; Spee, L.; Madderom, M.; Hemila, H. Commentaries on 'Vitamin C for preventing and treating the common cold' with responses from the review author. Evid. Based Child Health: A Cochrane Rev. J. 2008, 3, 723-728. [CrossRef]
Hemila, H. Vitamin C and common cold incidence: A review of studies with subjects under heavy physical stress. Int. J. Sports Med. 1996, 17, 379-383. Available online: https://helda.helsinki.fi/handle/10138/225881 (accessed on 14 December 2021). [CrossRef]
Hemila, H. Vitamin C intake and susceptibility to the common cold. Br. J. Nutr. 1997, 77, 59-72. [CrossRef]
Bates, C.J.; Schorah, C.J.; Hemila, H. Vitamin C intake and susceptibility to the common cold: Invited commentaries. Br. J. Nutr. 1998, 78, 857-866. [CrossRef]
Vorilhon, P.; Arpajou, B.; Vaillant Roussel, H.; Merlin, E.; Pereira, B.; Cabaillot, A. Efficacy of vitamin C for the prevention and treatment of upper respiratory tract infection: A meta-analysis in children. Eur. J. Clin. Pharmacol. 2019, 75, 303-311. [CrossRef]
Padhani, Z.A.; Moazzam, Z.; Ashraf, A.; Bilal, H.; Salam, R.A.; Das, J.K.; Bhutta, Z.A. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst. Rev. 2020, 4, CD013134. [CrossRef]
Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D'Antona, G. The long history of vitamin C: From prevention of the common cold to potential aid in the treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [CrossRef]
Ran, L.; Zhao, W.; Wang, J.; Wang, H.; Zhao, Y.; Tseng, Y.; Bu, H. Extra dose of vitamin C based on a daily supplementation shortens the common cold: A meta-analysis of 9 randomized controlled trials. Biomed. Res. Int. 2018, 2018,1837634. [CrossRef]
Li, R.; Guo, C.; Li, Y.; Liang, X.; Yang, L.; Huang, W. Therapeutic target and molecular mechanism of vitamin C-treated pneumonia: A systematic study of network pharmacology. Food Funct. 2020,11, 4765-4772. [CrossRef] [PubMed]
Hui, L.L.; Nelson, E.A.S.; Lin, S.L.; Zhao, J.V. The role of vitamin C in pneumonia and COVID-19 infection in adults with European ancestry: A Mendelian randomisation study. Eur. J. Clin. Nutr. 2021. [CrossRef] [PubMed]
Hemila, H.; Chalker, E. Meta-analysis on vitamin C and the common cold in children may be misleading. Eur. J. Clin. Pharmacol. 2019, 75,1747-1748. Available online: https://helda.helsinki.fi/handle/10138/318103 (accessed on 14 December 2021). [CrossRef]
Hemila, H.; Chalker, E. Vitamin C and the common cold in children: Severe flaws in the meta-analyses by Vorilhon et al. 2019. Available online: https://helda.helsinki.fi/handle/10138/333365 (accessed on 14 December 2021).
Vorilhon, P.; Arpajou, B.; Roussel, H.V.; Merlin, E.; Pereira, B.; Cabaillot, A. Retraction Note: Efficacy of vitamin C for the prevention and treatment of upper respiratory tract infection: A meta-analysis in children. Eur. J. Clin. Pharmacol. 2021, 77, 941. [CrossRef] [PubMed]
Hemila, H.; Chalker, E. Commentary: Vitamin C supplementation for prevention and treatment of pneumonia. Front Med (Lausanne) 2021, 7, 595988. [CrossRef]
Hemila, H.; Chalker, E. Commentary: The long history of vitamin C: From prevention of the common cold to potential aid in the treatment of COVID-19. Front. Immunol. 2021,12, 659001. [CrossRef]
Hemila, H. Shortcomings in the Vitamin C and Common Cold Meta-Analysis by Ran et al. 2018. PubPeer 2021 Mar. Available online: https://pubpeer.com/publications/077EAB95E9141BE6A455C30F0A688E (accessed on 14 December 2021).
Hemila, H.; Carr, A. Comment on "Therapeutic target and molecular mechanism of vitamin C-treated pneumonia: A systematic study of network pharmacology" by R. Li, C. Guo, Y. Li, X. Liang, L. Yang and W. Huang. Food Funct. 2021, 12, 1371-1372. [CrossRef] [PubMed]
Hemila, H.; Chalker, E. Assessment of Vitamin C Effects on Pneumonia and COVID-19 Using Mendelian Randomization: Analysis May Be Misleading. PubPeer 2021 Dec. Available online: https://pubpeer.com/publications/DEB2ADB9FB83E83795C95E8D1 FFEB7 (accessed on 14 December 2021).
Hemila, H. Do Vitamins C and E Affect Respiratory Infections? Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2006. Available online: https://helda.helsinki.fi/handle/10138/20335 (accessed on 14 December 2021).
Hemila, H. Vitamin C and the common cold. Br. J. Nutr. 1992, 67, 3-16. [CrossRef]
Hemila, H. Vitamin C supplementation and common cold symptoms: Factors affecting the magnitude of the benefit. Med Hypotheses 1999,52,171-178. Available online: https://helda.helsinki.fi/handle/10138/223761 (accessed on 14 December 2021). [CrossRef]
Hemila, H. Vitamin C supplementation and respiratory infections: A systematic review. Mil. Med. 2004,169, 920-925. [CrossRef]
Hemila, H.; Louhiala, P. Vitamin C may affect lung infections. J. R. Soc. Med. 2007,100, 495-498. [CrossRef]
Hemila, H.; Louhiala, P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013, CD005532. Available online: https://helda.helsinki.fi/handle/10138/225862 (accessed on 14 December 2021). [CrossRef]
Hemila, H.; Louhiala, P. Vitamin C for Preventing and Treating Pneumonia. Available online: http://www.mv.helsinki.fi/home/ hemila/CP (accessed on 14 December 2021).
Hemila, H. Vitamin C and common cold-induced asthma: A systematic review and statistical analysis. Allergy Asthma Clin. Immunol. 2013, 9, 46. [CrossRef]
Hemila, H. Vitamin C and infections. Nutrients 2017, 9, 339. [CrossRef]
Karlowski, T.R.; Chalmers, T.C.; Frenkel, L.D.; Kapikian, A.Z.; Lewis, T.L.; Lynch, J.M. Ascorbic acid for the common cold: A prophylactic and therapeutic trial. JAMA 1975, 231,1038-1042. [CrossRef] [PubMed]
Lewis, T.L.; Karlowski, T.R.; Kapikian, A.Z.; Lynch, J.M.; Shaffer, G.W.; George, D.A. A controlled clinical trial of ascorbic acid for the common cold. Ann. NY Acad. Sci. 1975,258, 505-512. [CrossRef] [PubMed]
Liberati, A. Thomas C Chalmers. Lancet 1996, 34,188. [CrossRef]
Dickersin, K. Thomas Clark Chalmers. JAMA 1996, 276, 656-657. [CrossRef]
Lau, J. Tributes to Thomas Chalmers. Ann. Intern. Med. 1996,124, 696. [CrossRef]
Huth, E.J. Tributes to Thomas Chalmers. Ann. Intern. Med. 1996,124, 696. [CrossRef]
Mulrow, C. Tributes to Thomas Chalmers. Ann. Intern. Med. 1996,124, 696. [CrossRef]
Knatterud, G.; Greenhouse, S.W. Tributes to Dr. Thomas, C. Chalmers. Control. Clin. Trials 1996,17, 473-475. [CrossRef]
Chalmers, T.C. Dr. Tom Chalmers, 1917-1995: The trials of a randomizer. Interview by Malcolm Maclure. CMAJ1996,155, 757-760. [PubMed]
Chalmers, T.C. Dr. Tom Chalmers, 1917-1995: The tribulations of a trialist. Interview by Malcolm Maclure. CMAJ 1996, 155, 986-988. [PubMed]
Sackett, D. A 1955 clinical trial report that changed my career. J. R. Soc. Med. 2010,103, 254-255. [CrossRef] [PubMed]
Dickersin, K.; Chalmers, F. Thomas C Chalmers (1917-1995): A pioneer of randomised clinical trials and systematic reviews. J. R. Soc. Med. 2015,108, 237-241. [CrossRef]
Smith, R.; Rennie, D. Evidence based medicine: An oral history. BMJ 2014, 348, g371. [CrossRef] [PubMed]
Chalmers, T.C. To the preceding article by H. Hemila. J. Clin. Epidemiol. 1996, 49,1085. [CrossRef]
Hemila, H. Vitamin C, the placebo effect, and the common cold: A case study of how preconceptions influence the analysis of results. J. Clin. Epidemiol. 1996, 49,1079-1084. Available online: https://helda.helsinki.fi/handle/10250/8082 (accessed on 14 December 2021). [CrossRef]
Hemila, H. Assessment of blinding may be inappropriate after the trial. Contemp. Clin. Trials. 2005, 26, 512-514. Available online: https://helda.helsinki.fi/handle/10138/228099 (accessed on 14 December 2021). [CrossRef] [PubMed]
Hemila, H. Analysis of clinical data with breached blindness. Stat. Med. 2006, 25,1434-1437. Available online: https://helda. helsinki.fi/handle/10138/228098 (accessed on 14 December 2021). [CrossRef]
Hemila, H. Assessment of the importance of double-blinding should be based on systematic reviews. J. Thromb. Haemost. 2008, 6,1247-1248. Available online: https://helda.helsinki.fi/handle/10138/228091 (accessed on 14 December 2021). [CrossRef]
Ludvigsson, J.; Hansson, L.O.; Tibbling, G. Vitamin C as a preventive medicine against common colds in children. Scand. J. Infect. Dis. 1977, 9, 91-98. [CrossRef]
Pitt, H.A.; Costrini, A.M. Vitamin C prophylaxis in marine recruits. JAMA 1979, 241, 908-911. [CrossRef] [PubMed]
Anderson, T.W.; Reid, D.B.; Beaton, G.H. Vitamin C and the common cold: A double-blind trial. Can. Med. Assoc. J. 1972, 107, 503-508.
Hemila, H. To the dissent by Thomas Chalmers. J. Clin. Epidemiol. 1996, 49,1087. Available online: https://helda.helsinki.fi/ handle/10138/225873 (accessed on 14 December 2021). [CrossRef]
Hendley, J.O. The common cold. In Cecil Textbook of Medicine, 20th ed.; Bennett, J.C., Blum, F., Eds.; Saunders: Philadelphia, PA, USA, 1996; pp. 1747-1749.
Hendley, J.O. The common cold. In Cecil Textbook of Medicine, 21th ed.; Goldman, L., Bennett, J.C., Eds.; Saunders: Philadelphia, PA, USA, 2000; pp. 1790-1793.
Hendley, J.O. The common cold. In Cecil Textbook of Medicine, 22th ed.; Goldman, L., Bennett, J.C., Eds.; Saunders: Philadelphia, PA, USA, 2004; pp. 1967-1969.
Gwaltney, J.M. The common cold. In Principles and Practice of Infectious Diseases, 1st ed.; Mandell, G.L., Douglas, R.G., Bennett, J.E., Eds.; Churchill Livingstone: New York, NY, USA, 1979; pp. 429-435.
Gwaltney, J.M. The common cold. In Principles and Practice of Infectious Diseases, 2nd ed.; Mandell, G.L., Douglas, R.G., Bennett, J.E., Eds.; Churchill Livingstone: New York, NY, USA, 1985; pp. 351-355.
Gwaltney, J.M. The common cold. In Principles and Practice of Infectious Diseases, 3rd ed.; Mandell, G.L., Douglas, R.G., Bennett, J.E., Eds.; Churchill Livingstone: New York, NY, USA, 1990; pp. 489-493.
Gwaltney, J.M. The common cold. In Principles and Practice of Infectious Diseases, 4th ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingstone: New York, NY, USA, 1995; pp. 561-566.
Cherry, J.D. The common cold. In Textbook of Pediatric Infectious Diseases, 2nd ed.; Feigin, R.D., Cherry, J.D., Eds.; Saunders: Philadelphia, PA, USA, 1987; pp. 155-161.
Cherry, J.D. The common cold. In Textbook of Pediatric Infectious Diseases, 3th ed.; Feigin, R.D., Cherry, J.D., Eds.; Saunders: Philadelphia, PA, USA, 1992; pp. 137-142.
Cherry, J.D. The common cold. In Textbook of Pediatric Infectious Diseases, 4th ed.; Feigin, R.D., Cherry, J.D., Eds.; Saunders: Philadelphia, PA, USA, 1998; pp. 128-133.
Food and Nutrition Board, National Research Council. Recommended Dietary Allowances, 9th ed.; National Academy of Sciences: Washington, DC, USA, 1980; pp. 72-82.
Food and Nutrition Board, Institute of Medicine. Evolution of Evidence for Selected Nutrients and Disease Relationships; National Academy Press: Washington, DC, USA, 2002; pp. 36-38. Available online: https://www.ncbi.nlm.nih.gov/books/NBK220648 (accessed on 14 December 2021). [CrossRef]
Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gntzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [CrossRef] [PubMed]
Mulrow, C.D.; Oxman, A.D. The Cochrane Handbook 3.0; The Cochrane Collaboration, Update Software: Oxford, UK, 1994.
Clarke, M.; Oxman, A.D. Cochrane Reviewers' Handbook 4.1; The Cochrane Library: Hoboken, NJ, USA, 2000; pp. 39-40.
Alderson, P; Green, S.; Higgins, J.P.T. Cochrane Reviewers' Handbook 4.2.2; The Cochrane Library: Oxford, UK, 2004; p. 52.
Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6; The Cochrane Library: Hoboken, NJ, USA,
2006; pp. 81-82.
Friedman, L.M.; Furberg, C.D.; DeMets, D.L. Fundamentals of Clinical Trials, 1st ed.; John Wright: Boston, MA, USA, 1982; pp. 59, 206.
Friedman, L.M.; Furberg, C.D.; DeMets, D.L. Fundamentals of Clinical Trials, 2nd ed.; PGN Publishing: Littleton, MA, USA, 1985; pp. 72, 279.
Friedman, L.M.; Furberg, C.D.; DeMets, D.L. Fundamentals of Clinical Trials, 3rd ed.; Springer: New York, NY, USA, 1998; pp. 83, 337.
Friedman, L.M.; Furberg, C.D.; DeMets, D.L. Fundamentals of Clinical Trials, 4th ed.; Springer: New York, NY, USA, 2010; pp. 120, 418. [CrossRef]
Weiss, N.S. Clinical Epidemiology, 1st ed.; University of Oxford: New York, NY, USA, 1986; p. 55.
Weiss, N.S. Clinical Epidemiology, 2nd ed.; University of Oxford: New York, NY, USA, 1996; p. 56.
Weiss, N.S. Clinical Epidemiology, 3rd ed.; University of Oxford: New York, NY, USA, 2006; p. 54.
Califf, R.M. Clinical trials. In Clinical and Translational Science: Principles of Human Research, 2nd ed.; Robertson, D., Williams, G.H., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 25-52. [CrossRef]
Califf, R.M. Large clinical trials and registries: Clinical research institutes. In Principles and Practice of Clinical Research, 2nd ed.; Gallin, J.I., Ognibene, F.P., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 237-263. [CrossRef]
Editorial. Vitamin C and the common cold. BMJ 1976,1, 606. [CrossRef]
Chalmers, T.C. Effects of ascorbic acid on the common cold: An evaluation of the evidence. Am. J. Med. 1975, 58, 532-536. [CrossRef]
American Medical Association, Council of Scientific Affairs. Vitamin preparations as dietary supplements and as therapeutic agents. JAMA 1987, 257,1929-1936. [CrossRef]
Bastian, H.; Glasziou, P.; Chalmers, I. Seventy-five trials and eleven systematic reviews a day: How will we ever keep up? PLoS Med. 2010, 7, e1000326. [CrossRef] [PubMed]
Hemilä, H. Thomas Chalmers, vitamin C and the common cold. J. R. Soc. Med. 2016,109, 46. [CrossRef]
Hemilä, H.; Herman, Z.S. Vitamin C and the common cold: A retrospective analysis of Chalmers' review. J. Am. Coll. Nutr. 1995,14,116-123. Available online: https://helda.helsinki.fi/handle/10138/42358 (accessed on 14 December 2021). [CrossRef] [PubMed]
Cowan, D.; Diehl, H.S.; Baker, A.B. Vitamins for the prevention of colds. JAMA 1942,120,1268-1271. [CrossRef]
Food and Nutrition Board, National Research Council. Recommended Dietary Allowances, 10th ed.; National Academy Press: Washington, DC, USA, 1989; pp. 115-125. [CrossRef]
Thurnham, D.I.; Bender, D.A.; Scott, J. Water-soluble vitamins. In Human Nutrition and Dietetics, 10th ed.; Garrow, J.S., James, W.P.T., Ralph, A., Eds.; Churchill Livingstone: London, UK, 2000; pp. 249-287.
Halsted, C.H. Water-soluble vitamins. In Human Nutrition and Dietetics, 9th ed.; Garrow, J.S., James, W.P.T., Eds.; Churchill Livingstone: London, UK, 1993; pp. 239-263.
Hamilton, E.M.N.; Whitney, E.N. Nutrition, Concepts and Controversies, 2nd ed.; West Publishing: New York, NY, USA, 1982; pp. 277-293.
Hamilton, E.M.N.; Whitney, E.N. Nutrition, Concepts and Controversies, 6th ed.; West Publishing: New York, NY, USA, 1994; pp. 403-429.
Shils, M.E.; Olson, J.A.; Shike, M. (Eds.) Modern Nutrition in Health and Disease, 8th ed.; Lea & Febiger: Malvern, PA, USA, 1994; pp. 652-661.
Hirsch, M.S.; Swartz, M.N. Antiviral agents. N. Engl. J. Med. 1980, 302, 949-953. [CrossRef] [PubMed]
Clarke, M. History of evidence synthesis to assess treatment effects: Personal reflections on something that is very much alive. J. R. Soc. Med. 2016,109,154-163. [CrossRef] [PubMed]
Sperber, S.J.; Hayden, F.G. Chemotherapy of rhinovirus colds. Antimicrob. Agents Chemother. 1988, 32, 409-419. [CrossRef] [PubMed]
Hemila, H. Many continuous variables such as the duration of the common cold should be analyzed using the relative scale. J. Clin. Epidemiol. 2016, 78,128-129. Available online: https://helda.helsinki.fi/handle/10138/173096 (accessed on 14 December 2021). [CrossRef]
Hemila, H. Duration of the common cold and similar continuous outcomes should be analyzed on the relative scale: A case study of two zinc lozenge trials. BMC Med. Res. Methodol. 2017,17, 82. [CrossRef] [PubMed]
Friedrich, J.O.; Adhikari, N.K.; Beyene, J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J. Clin. Epidemiol. 2011, 64, 556-564. [CrossRef]
Hemila, H. Vitamin C supplementation and common cold symptoms: Problems with inaccurate reviews. Nutrition. 1996, 12, 804-809. Available online: https://helda.helsinki.fi/handle/10138/225877 (accessed on 14 December 2021). [CrossRef]
Dykes, M.H.; Meier, P. Ascorbic acid and the common cold: Evaluation of its efficacy and toxicity. JAMA 1975, 231,1073-1079. [CrossRef]
Marks, H.M. A conversation with Paul Meier. Interview by Harry M Marks. Clin. Trials. 2004,1,131-138. [CrossRef]
Pincock, S. Paul Meier. Lancet 2011, 378, 978. [CrossRef]
Betts, K. Paul Meier: A man behind the method. Am. J. Public Health 2012,102, 2026-2029. [CrossRef]
Ritzel, G. Critical evaluation of vitamin C as a prophylactic and therapeutic agent in colds. Helv. Med. Acta. 1961, 28, 63-68. (In German). Available online: http://www.mv.helsinki.fi/home/hemila/T3.pdf (accessed on 14 December 2021). (In German).
Ritzel, G. Ascorbic acid and the common cold. JAMA 1976,235,1108. [CrossRef] [PubMed]
Coulehan, J.L.; Reisinger, K.S.; Rogers, K.D.; Bradley, D.W. Vitamin C prophylaxis in a boarding school. N. Engl. J. Med. 1974, 290, 6-10. [CrossRef]
Pauling, L. Ascorbic acid and the common cold: Evaluation of its efficacy and toxicity. Part I. Med. Trib. 1976,17,18-19. Available online: http://www.mv.helsinki.fi/home/hemila/pauling/Pauling1976MT_1.pdf (accessed on 14 December 2021).
Pauling, L. Medical establishment and vitamin C. In Vitamin C, the Common Cold, and the Flu; Freeman: San Francisco, CA, USA, 1976; pp. 121-138.
Pauling, L. Organized medicine and the vitamins. In How to Live Longer and Feel Better; Freeman: San Francisco, CA, USA, 1986; pp. 225-236.
Pauling, L. Ascorbic acid and the common cold. Part II. Medical Tribune 1976, 17, 37-38. Available online: http://www.mv. helsinki.fi/home/hemila/pauling/Pauling1976MT_2.pdf (accessed on 14 December 2021).
Hemila, H. Vitamin C supplementation and the common cold: Was Linus Pauling right or wrong? lnt. J. Vitam. Nutr. Res. 1997, 67, 329-335. Available online: https://helda.helsinki.fi/handle/10250/7980 (accessed on 14 December 2021). [PubMed]
Wilson, J.X.; Wu, F. Vitamin C in sepsis. Subcell. Biochem. 2012, 56, 67-83. [CrossRef] [PubMed]
Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Wegelin, J.A.; Brophy, D.; Ward, K.R.; Voelkel, N.F.; Fowler, A.A.; Natarajan, R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L20-L32. [CrossRef]
Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial. JAMA 2019, 322,1261-1270. [CrossRef]
Hemila, H.; Chalker, E. Reanalysis of the effect of vitamin C on mortality in the CITRIS-ALI trial: Important findings dismissed in the trial report. Front. Med. 2020, 7, 590853. [CrossRef]
Fowler, A.A. Vitamin C infusion for Treatment in Sepsis Induced Acute Lung Injury (CITRIS-ALI) [Trial Registration; Last Update 2019-10-15]. ClinicalTrials.gov NCT02106975. Available online: https://clinicaltrials.gov/ct2/show/NCT02106975 (accessed on 14 December 2021).
De Gruttola, V.; Fleming, T.; Lin, D.Y.; Coombs, R. Validating surrogate markers: Are we being naive? J. Infect. Dis. 1997, 175, 237-246. [CrossRef]
Temple, R. Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 1999,282, 790-795. [CrossRef]
Grimes, D.A.; Schulz, K.F. Surrogate end points in clinical research: Hazardous to your health. Obstet. Gynecol. 2005, 105,1114-1118. [CrossRef]
Institute of Medicine. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease; National Academies Press: Washington, DC, USA, 2010. [CrossRef]
Hemila, H.; Suonsyrja, T. Vitamin C for preventing atrial fibrillation in high risk patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2018,17, 49. [CrossRef]
Hemila, H.; Chalker, E. Vitamin C can shorten the length of stay in the ICU: A meta-analysis. Nutrients 2019,11, 708. [CrossRef]
Zabet, M.H.; Mohammadi, M.; Ramezani, M.; Khalili, H. Effect of high-dose ascorbic acid on vasopressor's requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94-100. [CrossRef]
Montori, V.M.; Guyatt, G.H. Intention-to-treat principle. CMAJ 2001,165,1339-1341.
McCoy, C.E. Understanding the intention-to-treat principle in randomized controlled trials. West. J. Emerg. Med. 2017, 18,1075-1078. [CrossRef] [PubMed]
DeMets, D.L.; Cook, T. Challenges of non-intention-to-treat analyses. JAMA 2019, 321,145-146. [CrossRef] [PubMed]
De Grooth, H.J.; Elbers, P.W.G.; Vincent, J.L. Vitamin C for sepsis and acute respiratory failure. JAMA 2020, 323, 792. [CrossRef]
Fowler, A.A.; Fisher, B.J.; Kashiouris, M.G. Vitamin C for sepsis and acute respiratory failure: Reply. JAMA 2020, 323, 792-793. [CrossRef] [PubMed]
Marik, P.E.; Payen, D. CITRIS-ALI: How statistics were used to obfuscate the true findings. Anaesth. Crit. Care Pain Med. 2019, 38, 575-577. [CrossRef]
Koenker, R. quantreg: Quantile Regression. 2021. Available online: https://CRAN.R-project.org/package=quantreg (accessed on 14 December 2021).
Hemila, H.; de Man, A.M.E. Vitamin C and COVID-19. Front. Med. 2021, 7, 559811. [CrossRef]
Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il'Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: The COVID A to Z randomized clinical trial. JAMA Netw. Open. 2021, 4, e210369. [CrossRef]
Hemila, H.; Carr, A.; Chalker, E. Vitamin C may increase the recovery rate of outpatient cases of SARS-CoV-2 infection by 70%: Reanalysis of the COVID A to Z randomized clinical trial. Front. Immunol. 2021,12, 674681. [CrossRef]
Villa, J.; Pannu, T.; McWilliams, C.; Kizer, C.; Rosenthal, R.; Higuera, C.; Patel, P. Results of preoperative screening for COVID-19 correlate with the incidence of infection in the general population: A tertiary care experience. Hosp. Pract. 2021, 49, 216-220. [CrossRef]
FDA approves first treatment for COVID-19 [FDA News Release October 22, 2020]. Available online: https://www.fda.gov/ news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 14 December 2021).
Brant, E.B.; Angus, D.C. Is high-dose vitamin C beneficial for patients with sepsis? JAMA 2019, 322, 1257-1258. [CrossRef] [PubMed]
Hemila, H. Biased editorial on vitamin C in JAMA. PubPeer 2020 October. Available online: https://pubpeer.com/publications/ 6C1E79E2B8EA7E2DEED9B46C2B717C (accessed on 14 December 2021).
Pauling, L. Evolution and the need for ascorbic acid. Proc. Natl. Acad. Sci. USA 1970, 67,1643-1648. [CrossRef]
Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685-3689. [CrossRef] [PubMed]
Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538-4542. [CrossRef] [PubMed]
Cameron, E.; Pauling, L. Experimental studies designed to evaluate the management of patients with incurable cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 6252. [CrossRef]
Cameron, E.; Pauling, L.; Leibovitz, B. Ascorbic acid and cancer: A review. Cancer Res. 1979, 39, 663-681.
Pauling, L. Vitamin C therapy of advanced cancer. N. Engl. J. Med. 1980, 302, 694. [CrossRef]
Pauling, L.; Herman, Z.S. Criteria for the validity of clinical trials of treatments of cohorts of cancer patients based on the Hardin Jones principle. Proc. Natl. Acad. Sci. USA 1989, 86, 6835-6837. [CrossRef]
Michos, E.D.; Cainzos-Achirica, M.C. Supplements for the treatment of mild COVID-19: Challenging health beliefs with science from A to Z. JAMA Netw. Open 2021, 4, e210431. [CrossRef]
Hemila, H. Misleading Editorial on the COVID A to Z trial. PubPeer 2021 February. Available online: https://pubpeer.com/ publications/7C275B4F3B2A74D33320D4D9C37AA3 (accessed on 14 December 2021).
Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. 2020,14, 367-382. [CrossRef]
Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004,140, 533-537. [CrossRef]
Anderson, T.W.; Suranyi, G.; Beaton, G.H. The effect on winter illness of large doses of vitamin C. Can. Med. Assoc. J. 1974, 111,31-36.
Kalil, A.C. Lack of benefit of high-dose vitamin C, thiamine, and hydrocortisone combination for patients with sepsis. JAMA 2020, 323, 419-420. [CrossRef] [PubMed]
Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: The VITAMINS randomized clinical trial. JAMA 2020, 323, 423-431. [CrossRef]
Hemila, H. Misleading Editorial on Vitamin C in JAMA. PubPeer 2020 October. Available online: https://pubpeer.com/ publications/07DCB1F79D50182367BF913CC69004 (accessed on 14 December 2021).
Hodges, R.E.; Hood, J.; Canham, J.E.; Sauberlich, H.E.; Baker, E.M. Clinical manifestations of ascorbic acid deficiency in man. Am. J. Clin. Nutr. 1971,24, 432-443. [CrossRef]
Hunt, C.; Chakravorty, N.K.; Annan, G.; Habibzadeh, N.; Schorah, C.J. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int. J. Vitam. Nutr. Res. 1994, 64, 212-219. Available online: http://www.mv.helsinki.fi/home/hemila/CP/Hunt1994ch.pdf (accessed on 14 December 2021). [PubMed]
Fain, O.; Mathieu, E.; Thomas, M. Scurvy in patients with cancer. BMJ 1998, 316,1661-1662. [CrossRef] [PubMed]
Teixeira, A.; Carrie, A.S.; Genereau, T.; Herson, S.; Cherin, P. Vitamin C deficiency in elderly hospitalized patients. Am. J. Med. 2001, 111, 502. [CrossRef]
Fain, O.; Paries, J.; Jacquart, B.; Le Moel, G.; Kettaneh, A.; Stirnemann, J.; Heron, C.; Sitbon, M.; Taleb, C.; Letellier, E.; et al. Hypovitaminosis C in hospitalized patients. Eur. J. Intern. Med. 2003,14, 419-425. [CrossRef] [PubMed]
Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin C deficiency in cancer patients. Palliat. Med. 2005,19,17-20. [CrossRef]
Gan, R.; Eintracht, S.; Hoffer, L.J. Vitamin C deficiency in a university teaching hospital. J. Am. Coll. Nutr. 2008, 27, 428-433. [CrossRef]
Raynaud-Simon, A.; Cohen-Bittan, J.; Gouronnec, A.; Pautas, E.; Senet, P.; Verny, M.; Boddaert, J. Scurvy in hospitalized elderly patients. J. Nutr. Health Aging 2010,14, 407-410. [CrossRef] [PubMed]
Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care. 2017, 21, 300. [CrossRef]
Hodges, R.E. What's new about scurvy? Am. J. Clin. Nutr. 1971, 24, 383-384. [CrossRef] [PubMed]
Ferron-Celma, I.; Mansilla, A.; Hassan, L.; Garcia-Navarro, A.; Comino, A.M.; Bueno, P.; Ferron, J.A. Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J. Surg. Res. 2009,153, 224-230. [CrossRef]
Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014,12, 32. [CrossRef]
Nabil Habib, T.; Ahmed, I. Early adjuvant intravenous vitamin C treatment in septic shock may resolve the vasopressor dependence. Int. J. Microbiol. Adv. Immunol. 2017, 5, 77-81. [CrossRef]
Galley, H.F.; Howdle, P.D.; Walker, B.E.; Webster, N.R. The effects of intravenous antioxidants in patients with septic shock. Free. Radic. Biol. Med. 1997, 23, 768-774. [CrossRef]
Schneider, A.; Markowski, A.; Momma, M.; Seipt, C.; Luettig, B.; Hadem, J.; Wilhelmi, M.; Manns, M.P.; Wedemeyer, J. Tolerability and efficacy of a low-volume enteral supplement containing key nutrients in the critically ill. Clin. Nutr. 2011, 30, 599-603. [CrossRef] [PubMed]
Hemila, H.; Kaprio, J. Modification of the effect of vitamin E supplementation on the mortality of male smokers by age and dietary vitamin C. Am. J. Epidemiol. 2009,169, 946-953. [CrossRef]
Hemila, H.; Kaprio, J. Vitamin E supplementation and pneumonia risk in males who initiated smoking at an early age: Effect modification by body weight and dietary vitamin C. Nutr. J. 2008, 7, 33. [CrossRef]
Sample Size Calculator, ClinCalc. Available online: https://clincalc.com/stats/samplesize.aspx (accessed on 14 December 2021).
Hess, A.F. Scurvy: Past and Present; Lippincott: Philadelphia, PA, USA, 1920; pp. 88, 99. Available online: https://archive.org/ details/b29823778/page/n4/mode/2up (accessed on 14 December 2021).
Hess, A.F. Diet, nutrition and infection. N. Engl. J. Med. 1932, 207, 637-648. [CrossRef]
Clausen, S.W. The influence of nutrition upon resistance to infection. Physiol. Rev. 1934,14, 309-350. [CrossRef]
Robertson, E.C. The vitamins and resistance to infection. Medicine 1934,13,190-206. [CrossRef]
Hochwald, A. Vitamin C in the treatment of croupous pneumonia. Dtsch. Med. Wochensch. R 1937, 63,182-184. Available online: http://www.mv.helsinki.fi/home/hemila/T8.pdf (accessed on 14 December 2021). (In German). [CrossRef]
Bohnholtzer, E. Contribution to the question of pneumonia treatment with vitamin C. Dtsch. Med. Wochenschr 1937, 63,1001-1003. Available online: http://www.mv.helsinki.fi/home/hemila/T7.pdf (accessed on 14 December 2021). (In German). [CrossRef]
Glazebrook, A.J.; Thomson, S. The administration of vitamin C in a large institution and its effect on general health and resistance to infection. J. Hyg. 1942, 42,1-19. [CrossRef] [PubMed]
Klenner, F.R. Virus pneumonia and its treatment with vitamin C. South. Med. Surg. 1948,110, 36-38. [PubMed]
Abbasy, M.A.; Harris, L.J.; Hill, N.G. Vitamin C and infection: Excretion of vitamin C in osteomyelitis. Lancet 1937, 230,177-180. [CrossRef]
Ruskin, S.L. Calcium cevitamate in the treatment of acute rhinitis. Ann. Otol. Rhinol. Laryngol. 1938, 47, 502-511. [CrossRef]
Markwell, N.W. Vitamin C in the prevention of colds. Med. J. Aust. 1947, 2, 777-778. [CrossRef]
Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365-406. [CrossRef]
Rice, M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000, 23, 209-216. [CrossRef]
Mandl, J.; Szarka, A.; Banhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009,157,1097-1110. [CrossRef]
May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013,19, 2068-2083. [CrossRef] [PubMed]
Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016,22, 463-493. [CrossRef]
Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015,19, 418. [CrossRef]
Kumar, D.; Mains, R.E.; Eipper, B.A. 60 YEARS OF POMC: From POMC and a-MSH to PAM, molecular oxygen, copper, and vitamin C. J. Mol. Endocrinol. 2016, 56, T63-T76. [CrossRef]
Young, J.I.; Zuchner, S.; Wang, G. Regulation of the epigenome by vitamin C. Annu. Rev. Nutr. 2015, 35, 545-564. [CrossRef] [PubMed]
Lee Chong, T.; Ahearn, E.L.; Cimmino, L. Reprogramming the epigenome with vitamin C. Front. Cell. Dev. Biol. 2019, 7,128. [CrossRef]
Brabson, J.P.; Leesang, T.; Mohammad, S.; Cimmino, L. Epigenetic regulation of genomic stability by vitamin C. Front. Genet. 2021,12, 675780. [CrossRef] [PubMed]
Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020,159, 37-43. [CrossRef]
Manning, J.; Mitchell, B.; Appadurai, D.A.; Shakya, A.; Pierce, L.J.; Wang, H.; Nganga, V.; Swanson, P.C.; May, J.M.; Tantin, D.; et al. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013,19, 2054-2067. [CrossRef] [PubMed]
Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9,1211. [CrossRef]
Ang, A.; Pullar, J.M.; Currie, M.J.; Vissers, M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018, 46,1147-1159. [CrossRef]
Padayatty, S.J.; Levine, M. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer. Ann. Intern. Med. 2014,160, 654. [CrossRef]
Merchant, A.T.; Curhan, G.; Bendich, A.; Singh, V.N.; Willett, W.C.; Fawzi, W.W. Vitamin intake is not associated with community- acquired pneumonia in U.S. men. J. Nutr. 2004,134, 439-444. [CrossRef] [PubMed]
Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids; National Academy Press: Washington, DC, USA, 2000; pp. 95-185. [CrossRef]
Rowe, S.; Carr, A.C. Global vitamin C status and prevalence of deficiency: A cause for concern? Nutrients 2020, 12, 2008. [CrossRef]
Anderson, T.W.; Beaton, G.H.; Corey, P.; Spero, L. Winter illness and vitamin C: The effect of relatively low doses. Can. Med. Assoc. J. 1975, 112, 823-826.
Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians' Health Study II randomized controlled trial. JAMA 2008, 300, 2123-2133. [CrossRef]
Schorah, C.J.; Newill, A.; Scott, D.L.; Morgan, D.B. Clinical effects of vitamin C in elderly inpatients with low blood-vitamin C levels. Lancet 1979,1, 403-405. [CrossRef]
Apple, R.D. Vitamania: Vitamins in American Culture; Rutgers University Press: Hoboken, NJ, USA, 1996.
Gratzer, W. Fruits and nuts. Nature 1996, 383, 589-590. [CrossRef]
Kinsey, J.D. Vitamania: Vitamins in American Culture by Rima, D. Apple. J. Consum. Aff. 1997, 31, 376-380.
Whorton, J.C. Vitamins and science. Pharm. History 1995, 37,153-154.
Jefferson, J.W. Vitamania: Vitamins in American Culture by Rima D. Apple. Wisconsin Mag. History 1997, 80, 318-319.
White, P.L. Megavitamin therapy. JAMA 1975, 234, 807. [CrossRef]
Pauling, L. Megavitamin therapy. JAMA 1976, 235, 598. [CrossRef]
Goodwin, J.S.; Tangum, M.R. Battling quackery: Attitudes about micronutrient supplements in American academic medicine. Arch. Intern. Med. 1998,158, 2187-2191. [CrossRef]
Herbert, V.; Jacob, E. Destruction of vitamin B12 by ascorbic acid. JAMA 1974,230, 241-242. [CrossRef]
Newmark, H.L.; Scheiner, M.S.; Marcus, M.; Prabhudesai, M. Stability of vitamin B12 in the presence of ascorbic acid. Am. J. Clin. Nutr. 1976, 29, 645-649. [CrossRef]
Newmark, H.L.; Scheiner, J.M.; Marcus, M.; Prabhudesai, M. Ascorbic acid and vitamin B12. JAMA 1979, 242, 2319-2320. [CrossRef] [PubMed]
White, P.L. Megavitamin this and megavitamin that. JAMA 1975, 233, 538-539. [CrossRef] [PubMed]
Jukes, T.H. Megavitamin therapy. JAMA 1975, 233, 550-551. [CrossRef] [PubMed]
Sackler, A.M. Megavitamin therapy. JAMA 1975, 234, 806. [CrossRef]
Swann, J.P. The history of efforts to regulate dietary supplements in the USA. Drug Test. Anal. 2016, 8, 271-282. [CrossRef]
Goodwin, J.S.; Goodwin, J.M. Failure to recognize efficacious treatments: A history of salicylate therapy in rheumatoid arthritis. Perspect. Biol. Med. 1981,25, 78-92. [CrossRef]
Goodwin, J.S.; Goodwin, J.M. The tomato effect: Rejection of highly efficacious therapies. JAMA 1984, 251, 2387-2390. [CrossRef]
Richards, E. The politics of therapeutic evaluation: The vitamin C and cancer controversy. Soc. Stud. Sci. 1988, 18,653-701. [CrossRef]
Richards, E. Vitamin C and Cancer: Medicine or Politics? St. Martins Press: New York, NY, USA, 1991. [CrossRef]
Segerstrale, U. Beleaguering the cancer establishment: Vitamin C and cancer: Medicine or politics? Science 1992, 255, 613-615. [CrossRef]
Huxtable, R.J. Vitamin C and cancer: Medicine or politics? Trends Pharmacol. Sci. 1992,13, 82-83. [CrossRef]
Galloway, J. Crusades and rackets. Nature 1991, 353,125. [CrossRef]
Herxheimer, A. Challenge for clinical trialists. BMJ 1991, 303,1076. [CrossRef]
McCormick, W.J. Cancer: A collagen disease, secondary to a nutritional deficiency. Arch. Pediatr. 1959, 76,166-171. [PubMed]
Jaffey, M. Vitamin C and cancer: Examination of the Vale of Leven trial results using broad inductive reasoning. Med. Hypotheses. 1982, 8, 49-84. [CrossRef]
Creagan, E.T.; Moertel, C.G.; O'Fallon, J.R.; Schutt, A.J.; O'Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer: A controlled trial. N. Engl. J. Med. 1979, 301, 687-690. [CrossRef]
Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O'Connell, M.J.; Ames, M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: A randomized double-blind comparison. N Engl. J. Med. 1985, 312,137-141. [CrossRef]
Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704-3709. [CrossRef]
Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011, 2, 78-88. [CrossRef]
Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105,11105-11109. [CrossRef]
Parrow, N.L.; Leshin, J.A.; Levine, M. Parenteral ascorbate as a cancer therapeutic: A reassessment based on pharmacokinetics. Antioxid Redox Signal. 2013,19, 2141-2156. [CrossRef]
Wohlrab, C.; Phillips, E.; Dachs, G.U. Vitamin C transporters in cancer: Current understanding and gaps in knowledge. Front. Oncol. 2017, 7, 74. [CrossRef]
Cimmino, L.; Neel, B.G.; Aifantis, I. Vitamin C in stem cell reprogramming and cancer. Trends Cell Biol. 2018, 28, 698-708. [CrossRef] [PubMed]
Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic acid in cancer treatment: Let the Phoenix fly. Cancer Cell 2018, 34, 700-706. [CrossRef]
Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271-282. [CrossRef] [PubMed]
Mikkelsen, S.U.; Gillberg, L.; Lykkesfeldt, J.; Grunbsk, K. The role of vitamin C in epigenetic cancer therapy. Free Radic. Biol. Med. 2021,170,179-193. [CrossRef] [PubMed]
Padayatty, S.J.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Hoffer, L.J.; Levine, M. Intravenously administered vitamin C as cancer therapy: Three cases. CMAJ 2006,174, 937-942. [CrossRef]
Hoffer, L.J.; Robitaille, L.; Zakarian, R.; Melnychuk, D.; Kavan, P; Agulnik, J.; Cohen, V.; Small, D.; Miller, W.H. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: A phase I-II clinical trial. PLoS ONE 2015, 10, e0120228. [CrossRef]
Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [CrossRef]
Davis, C.; Naci, H.; Gurpinar, E.; Poplavska, E.; Pinto, A.; Aggarwal, A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: Retrospective cohort study of drug approvals 2009-13. BMJ 2017, 359, j4530. [CrossRef] [PubMed]
Prasad, V. Do cancer drugs improve survival or quality of life? BMJ 2017, 359, j4528. [CrossRef] [PubMed]
Cohen, D. Cancer drugs: High price, uncertain value. BMJ 2017, 359, j4543. [CrossRef]
Naci, H.; Davis, C. Inappropriate use of progression-free survival in cancer drug approvals: New drugs should be judged on overall survival or quality of life, preferably both. BMJ 2020, 368, m770. [CrossRef]
Knipschild, P.; Kleijnen, J.; ter Riet, G. Belief in the efficacy of alternative medicine among general practitioners in The Netherlands. Soc. Sci. Med. 1990, 31, 625-626. [CrossRef]
National Center for Complementary and Integrative Health. Complementary, Alternative, or Integrative Health: What's In a Name? Available online: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a- name (accessed on 14 December 2021).
National Center for Complementary and Integrative Health. Flu and Colds: In Depth. Available online: https://www.nccih.nih. gov/health/flu-and-colds-in-depth (accessed on 14 December 2021).
Wieland, L.S.; Manheimer, E.; Berman, B.M. Development and classification of an operational definition of complementary and alternative medicine for the Cochrane Collaboration. Altern. Ther. Health Med. 2011,17, 50-59.
Louhiala, P.; Hemila, H. Can CAM treatments be evidence-based? Focus Altern. Complement. Ther. 2014,19, 84-89. Available online: https://helda.helsinki.fi/handle/10138/228056 (accessed on 14 December 2021). [CrossRef]
Vitamin C 2X reduction in colds if person has been exercising a lot and/or in cold weather- Cochrane - 2004
VITAMIN C FOR PREVENTING AND TREATING THE COMMON COLD
The Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No.: CD000980.pub2. DOI: 10.1002/14651858.CD000980.pub2.
Douglas RM, Hemila H, D'Souza R, Chalker EB, Treacy B
Main Results
Twenty-nine trial comparisons involving 11,077 study participants contributed to the metaanalysis on the relative risk (RR) of developing a cold while taking prophylaxis.
The pooled RR was 0.96 (95% CI 0.92 to 1.00).
A subgroup of six trials that involved a total of 642 marathon runners, skiers, and soldiers on sub-arctic exercises reported a pooled RR of 0.50 (95%CI 0.38 to 0.66).*Thirty comparisons that involved 9,676 respiratory episodes contributed to the metaanalysis on common cold duration during prophylaxis . A consistent benefit was observed, representing a reduction in cold duration of 8% (95% CI 3% to 13%) for adult participants and 13.5% (95% CI 5% to 21%) for child participants.
Reviewers' conclusions
The failure of vitamin C supplementation to reduce the incidence of colds in the normal population indicates that routine mega-dose prophylaxis is not rationally justified for community use. But evidence shows that it could be justified in persons exposed to brief periods of severe physical exercise and/or cold environments. Also, the consistent and statistically significant small benefits on duration and severity for those using regular vitamin C prophylaxis indicates that vitamin C plays some role in respiratory defence mechanisms. The trials in which vitamin C was introduced at the onset of colds as therapy did not show any benefit in doses up to 4 grams daily, but one large trial reported equivocal benefit from an 8 gram therapeutic dose at onset of symptoms.
📄 Download the PDF from VitaminDWiki
